
Also, Tekmira will collaborate with Bristol-Myers Squibb, Helicos Biosciences appoints Steve Lombardi CEO, more...


Also, Tekmira will collaborate with Bristol-Myers Squibb, Helicos Biosciences appoints Steve Lombardi CEO, more...

Actavis Totowa LLC, the US subsidiary of the generic drug manufacturer Actavis Group, is announcing a voluntary recall to the retail level of all drug products manufactured at its Little Falls, New Jersey, facility. This is a precautionary, voluntary action by Actavis following an inspection conducted by the Food and Drug Administration earlier this year.

Also, Ortho Biotech recalls one lot of "Procrit" due to cracked vials, more appointments at Actavis, more...

The House Committee on Energy and Commerce issued a revised discussion draft to the Food and Drug Administration Globalization Act of 2008.

Also, Bilcare Global Clinical Supplies opens new headquarters in San Francisco, Sigurdur Oli Olafsson named CEO of Actavis Group, more...

CMOs expand capacity and capabilities in high-potency manufacturing to meet strong demand for cytotoxic and other potent drugs.

This year's meeting of the Controlled Release Society unveiled a plethora of research insights.

Legislative decisions to increase Medicare's formulary may lead to a fight over drug approvals.

To meet the demands of early-stage development, contract research organizations can evaluate various dosage-form options. The author examines various methods of capsule filling, including binary blends.
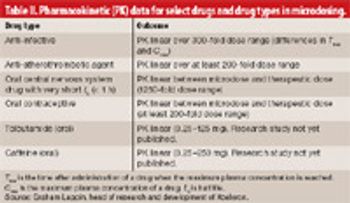
J. Scott Tarrant, executive vice-president of Xceleron, explains the role of microdosing in drug development. He describes how microdose data can be used to predict pharmacological dose absorption, distribution, metabolism, and excretion/pharmacokinetic outcomes using accelerator mass spectrometry.
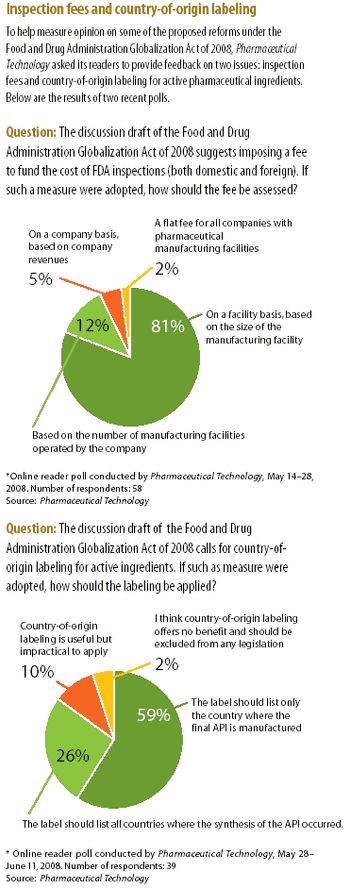
The high-profile case of contaminated heparin from a Chinese supplier has intensified the debate on the effectiveness of FDA's process for inspecting foreign drug-manufacturing facilities. The article examines proposed legislative and regulatory reforms and actions taken by the agency to improve drug-import safety.

Also, Cobra Biomanufacturing to extend collaboration and form joint venture with ViroMed, Epix Pharmaceuticals CEO resigns, more...

Pharmaceutical companies developing new drug candidates for Hepatitis C virus infection now can test their compounds with a novel culture system that mimics the biology of HCV infection in humans.

Senator Sherrod Brown sent a letter to Richard T. Clark, president and chief executive officer of Merck (Whitehouse Station, NJ), to ask for information about the company's reliance on global outsourcing for the manufacture of pharmaceutical ingredients and finished products.

Also, Niro changes its name to GEA Process Engineering; Frances K. Heller joins Exelixis as executive vice-president of business development, more...

3M Drug Delivery Systems has successfully designed a proof of concept device using a solid microstructured transdermal system for the systemic delivery of high-potency pharmaceuticals. The technology was showcased at a poster session at the annual meeting of the Controlled Release Society held this week in New York City.

Also, Roche to end HIV/AIDS research program, WuXi PharmaTech makes appointments, more...

Also, Catalent Pharma Solutions to collaborate with One World Design and Manufacturing Group, Bioheart appoints Howard J. Leonhardt as CEO, more...

The European Pharmacopoeia Commission adopted revised monographs for heparin calcium and heparin sodium to strengthen the level of testing required for quality control.

Also, Covance and WuXi PharmaTech to form contract research joint venture in China, Covidien makes appointments to its Pharmaceutical Products and Imaging Solutions businesses, more...

Molecular Profiles created a poster that demonstrates the efficacy of its "nanoPASS" (nanoscale predictive analytical screening solution) technique to characterize an active pharmaceutical ingredient's (API) surface energy.
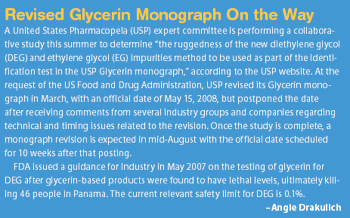
Drugmakers seeking to block the activity of a protein may have a new strategy at their disposal.

This review article explains how self-emulsifying drug delivery systems can increase the solubility and bioavailability of poorly soluble drug.
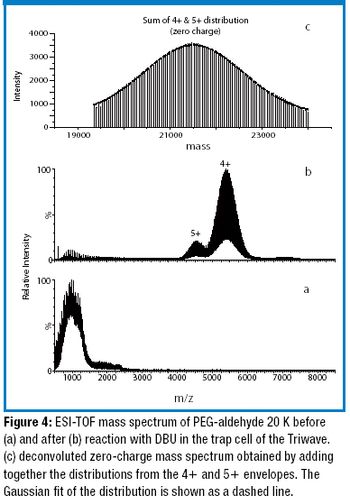
The authors developed a method to accurately measure the average molecular weight of large poly(ethylene glycols) (PEGs) using ion-mobility time-of-flight mass spectrometry coupled with gas-phase ion–molecule reactions.

A roundtable with John Doney, Jiao Yang, Hans Baer, and Elena Draganoiu.