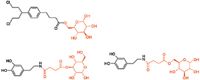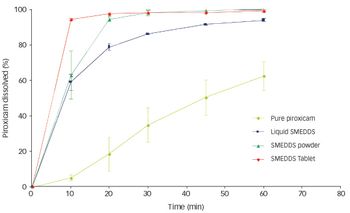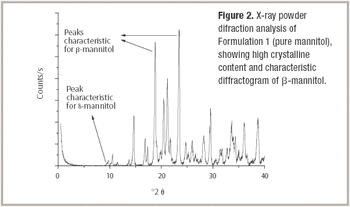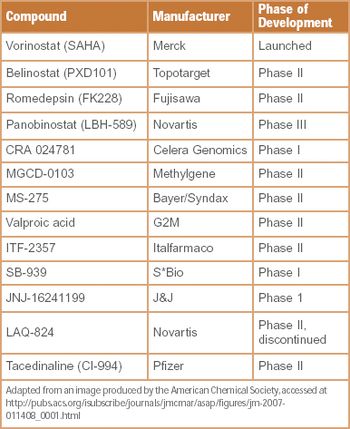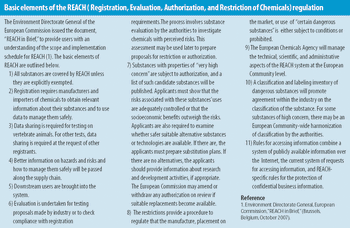
Our company is involved in developing and manufacturing APIs that can be utilized with drug-eluting stents (DES). Despite ensuring constancy in pharmaceutical composition, we are experiencing issues with variations in drug release during in vitro studies. We are working closely with a stent manufacturer to develop the system, but could surface analysis techniques investigate the problem further?