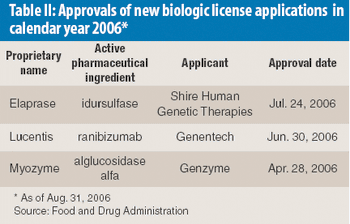
The authors investigated the influence of various particle size fractions of Tamarind seed polyose (TSP) on indomethacin (IND) release from matrix tablets. They assessed the TSP fractions for swelling, density, and flow properties and the IND matrix tablets for tensile strength, friability, and release profile. Release kinetics was evaluated using Higuchi and Peppas equations. The density and flow properties showed that the size fraction affects the suitability of TSP as an entrapment polymer. The release profile showed that the release of IND from TSP matrix is swelling dependent, thereby affecting the kinetics of release.