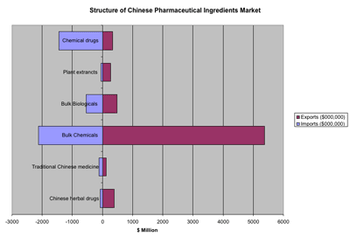
The sixth CPhI China exhibition, presented June 27?29 in Shanghai, offered a showcase for the explosive growth of the Chinese pharmaceutical sector.

The sixth CPhI China exhibition, presented June 27?29 in Shanghai, offered a showcase for the explosive growth of the Chinese pharmaceutical sector.

University of Buffalo (Buffalo, NY, www.buffalo.edu) researchers have developed a drug delivery system that uses an external magnetic field to guide drug-filled nanocarriers to cultured tumor cells.

Biopharmaceutical company Lipoxen PLC (London, UK) has developed a Hepatitis E vaccine using its novel vaccine delivery technology "ImuXen," which the company claims to be easy to manufacture. According to the company, the proprietary liposomal formulation method delivers vaccine materials to the immune system in a manner designed to emulate the response of a natural encounter with the infection agent.
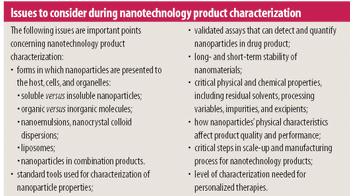
FDA is conducting laboratory research to understand better the ability of preclinical screening tests to identify potential risks and toxicities of nanotechnology-based drugs.

Select large custom manufacturers expand capacity, private equity firms buy companies in transition, and players from India and China build their positions.

This article summarizes changes to the Akers–Agalloco aseptic processing risk analysis model (first presented in Pharmaceutical Technology's November 2005 issue) as well as some of the underlying thinking behind the revision. The simplified model makes the method easier to use because of its greater flexibility of environmental control practice. It maintains the emphasis on human activity as the primary consideration in risk management for aseptic processing.

China is on the rise as a center for pharmaceutical R&D, but companies are still getting their footing for operating in China and the services industry has some maturing to do.

The hydrophilic matrix system continues to be the most popular and widely used strategy to achieve extended drug release. Hypromellose (hydroxypropylmethylcellulose [HPMC]) is typically the polymer of choice for the rate-controlling carrier in these systems.

Preformulation represents an early-stage opportunity to facilitate the eventual movement of a drug substance into a commercial product. Strategies to optimize the preformulation process were outlined by Harry Brittain, institute director for the Center for Pharmaceutical Physics (Milford, NJ). He spoke at the PharmTech Annual Event in Somerset, New Jersey this week.

RNAi therapeutics company SR Pharma plc (London, UK) has developed a process that allows it proprietary liposomal-based siRNA formulations ?AtuRNAi? drugs to be stored at room temperature and reconstituted in one step.

Recombinomics (Pittsburgh, PA) is again urging the World Health Organization to fully release all H5N1 avian influenza sequences, claiming their release would improve the selection of vaccines by helping scientists to identify the origin of the isolates and predict sequence changes.

I always suspected that our purchasing manager had agreed to this just to save money . . .

Predictable outcomes lead to greater manufacturing efficiency and speed time to value.
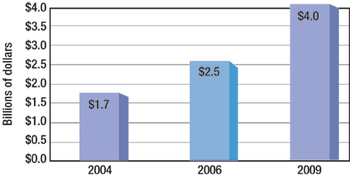
CMOs account for 20–30% of biopharm production. Big Pharma also is filling the biologics supply chain.
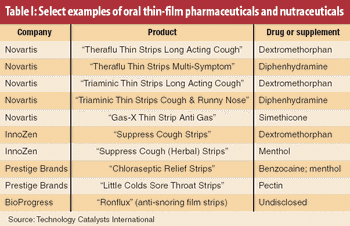
The biggest single recent trend in outsourcing solid-dosage processing has been the movement toward discovery and synthesis of more potent active pharmaceutical ingredients.
The construction of a new oral solid form (OSF) plant is an important decision and a real challenge. The team in charge of the basic conceptual design has to ensure that the new plant will be up-to-date and efficient not only at start-up, but for the next 15–20 years. This means that the project must be able to adjust to capacity changes, product changes and technology changes. It sometimes seems like an impossible challenge.
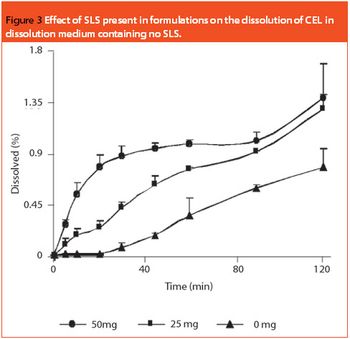
Bioequivalence with the reference product is the only reliable measure of demonstrating the therapeutic equivalence of a generic product to the innovator product. Systematic and comprehensive innovator product characterization can be used to make generic product development easier. This involves characterization of API and quantification of the critical excipients. The latter contributes towards performance of the final dosage form. This article describes the capsule formulation of a poorly water-soluble drug, celecoxib, which contains sodium lauryl sulphate as a critical excipient. The importance of a decoding process aimed at developing a generic product that matches the innovator formulation in a discriminating dissolution method is demonstrated.

On April 28, the US Food and Drug Administration's Center for Drug Evaluation and Research (Rockville, MD) issued a Warning Letter to Pliva Hrvatska d.o.o., a subsidiary of Pliva d.d. (Zagreb, Croatia).

Animal testing and accounting can both be hazardous.
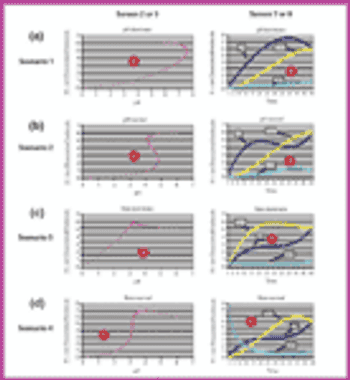
Using Bezier curves, an experimental process controller has been developed for biosynthesis applications in which the inactivity of a pH-sensitive enzyme must be decreased. By taking into account various control scenarios of pH and growth rate, as well as the physical and chemical characteristics of the environment, a suitable human-machine interface can be developed.
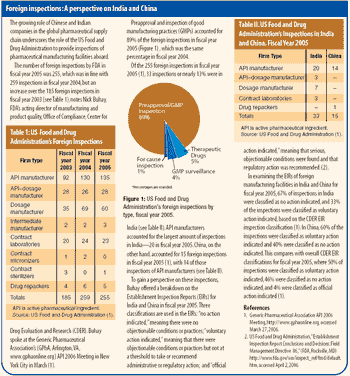
Indian suppliers of active pharmaceutical ingredients and dosage formulations expand in India, the United States, and Europe.

Manufacturing and formulation innovation spurs drug develop-ment, but raises new safety and quality issues.
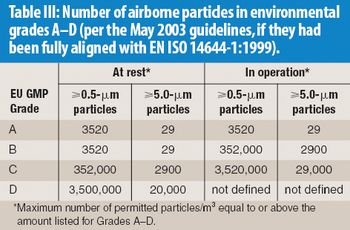
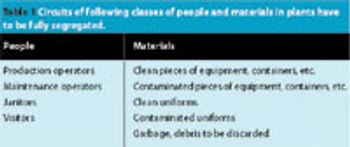
The author explores the importance of the proposals to revise Annex 1 of the EU GMPs in the context of the desire for science-based, internationally respected GMPs. Commentary also is provided about the relationship between this annex and CEN–ISO cleanroom standards.

This review article discusses orally disintegrating tablets and their manufacturing technologies, development issues, and future trends.
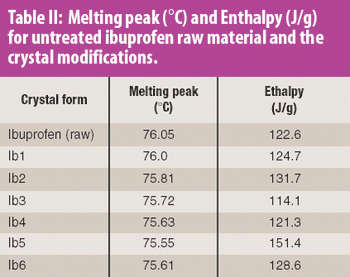
The common crystal form of ibuprofen was changed to optimize processing and manufacturing properties. Six modified crystal forms were prepared and assessed for dissolution, morphology, particle size, density, thermal characteristics, powder x-ray diffractometry, flow properties, and tabletability.