
Princeton, NJ (Nov. 30)-Rockwood Holdings, Inc. agreed to sell its Group Novasep subsidiary for EUR 425 million ($567 million) to a consortium of buyers consisting of Glide Buyout Partners BV, Banexi Capital, and Group Novasep management.

Princeton, NJ (Nov. 30)-Rockwood Holdings, Inc. agreed to sell its Group Novasep subsidiary for EUR 425 million ($567 million) to a consortium of buyers consisting of Glide Buyout Partners BV, Banexi Capital, and Group Novasep management.

Dublin, OH (Nov. 30)-Cardinal Health has announced plans to divest its Pharmaceutical Technologies and Services (PTS) segment, "a business that manufactures or packages 100 billion doses of medication every year for pharmaceutical and biotech firms, employs approximately 10,000 at more than 30 facilities worldwide and generates $1.8 billion in revenue," according to a company statement.

Pharmaceutical companies ally with specialty companies and research organizations to advance biocatalysis.

Makers of injectable drugs can increase their market share and stay competitive by devising a product life-cycle management strategy.

We really knew what we were doing-until we opened the column for a routine repacking.
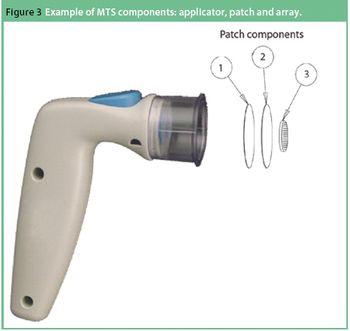
The needle and syringe have long been the standard delivery technology for vaccines. However, a confluence of market factors is driving new interest in alternative delivery systems that hold the potential to meet one or more of the following goals: improved antigen utilization, higher quality immune response, better stability and improved patient acceptance. Of particular interest are microneedle systems, otherwise referred to as microstuctured transdermal systems (MTS), that provide for targeted delivery of the vaccine formulation directly to antigen-presenting cells within the epidermis. This article provides a brief overview of MTS technology with an emphasis on solid-coated MTS for vaccine delivery.

EFPIA's 'Mock P.2' document aims to show how the role of 'quality risk management' and process analytical technology as an enabler for quality by design can be presented in a common technical document format. This article summarizes the main features of this document, and explains the key concepts and principles used.

London (Nov. 22)-The European Medicines Agency reports a defect in some vials of Herceptin (trastuzumab), the anticancer treatment by Roche, which have been distributed in Europe. As a result, The EMEA's Committee for Medicinal Products for Human Use outlined a plan, formulated in conjunction with Roche, for the visually reinspecting and replacing defective vials.

Grass allergic patients in Germany are the first to benefit from new vaccine

Research Triangle Park, NC (Nov. 13)-Eisai Inc. broke ground here for a new pharmaceutical production and formulation research and development facility for parenteral oncology drugs.

AAPS, San Antonio (Oct. 31)-Excipient manufacturers are raising concerns over recently adopted European guidelines, set to become effective January 1, 2007, which provide a framework and approach for dealing with genotoxic impurities in new active substances.

Philadelphia (Oct.24)?Failures to review batch failures and unexplained discrepancies are the leading cause of Food and Drug Administration Form 483 observations and Warning Letter citations issued to pharmaceutical companies.

Mid-size regional players have little chance for success in an industry increasingly dominated by large global players.

AstraZeneca's purchase of Cambridge Antibody Technology and Merck's acquisitions of GlycoFi and Abmaxis are the latest efforts by pharmaceutical majors to build critical mass in biologics capabilities.

Yet again, FDA's ability to regulate drugs is under fire. At the core of this latest round of scrutiny is whether the agency has the resources to properly control the safety of new nanotech-based drug products.
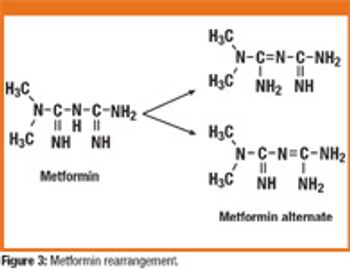
The correlation between swab assay results and visible-residue limits (VRLs) for cleaning validation was examined. Previously completed validation studies were reviewed to compare swab results with recently determined VRLs. A current cleaning validation study evaluated both swab testing and VRL. Unexpected swab results led to an investigation, which showed the value of establishing the VRL in conjunction with swab recoveries for cleaning validation programs.

Mid-size regional players have little chance for success in an industry increasingly dominated by large global players.

Here's to all the difficult, out-of-touch, and irresponsible coworkers that make our workplace interesting.
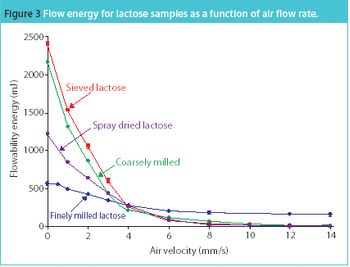
The trend towards developing pharmaceutical products and their manufacturing processes in tandem supports optimized production. Such developments rely on gathering process-relevant information at an early stage and being able to draw on past and current processing experience. Here, we discuss how powder rheometers can make a real difference in building a database of powder properties and removing subjectivity.
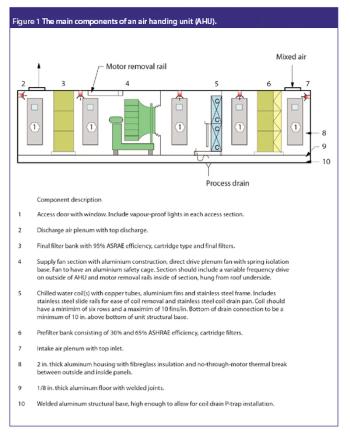
Clean rooms are critical areas in bio/pharma facilities, and it is essential that users are responsible for their care and upkeep, and familiarize themselves with the relevant regulations.

The ¤9.5 billion therapeutic antibody market comprises over a dozen antibodies that have been generated using recombinant genetic methods developed over the last 20 years.
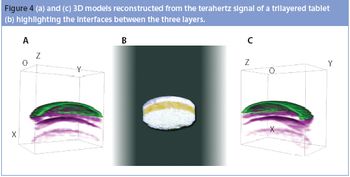
This article investigates pharmaceutical applications of terahertz technology, specifically using techniques for solid dosage form analysis such as pulsed spectroscopy (to generate physical information and detect API changes) and pulsed imaging (to locate formulation impurities, and regulate tablet coating quality and thickness).

Robin Hwang, a senior principal scientist at Amgen (Thousand Oaks, CA), led the team that developed the first commercial disposable auto-injector for a biopharmaceutical: a prefilled three-step "SureClick" for delivering Enbrel (etanercept), a treatment for autoimmune diseases.

Increased treatment using combination therapies

CPhI success