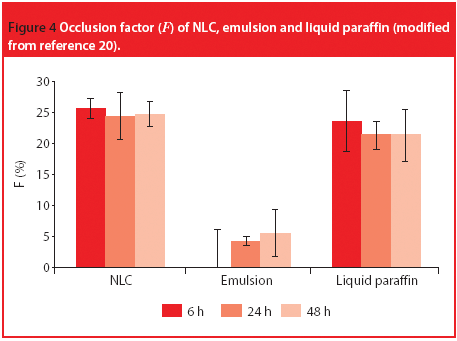
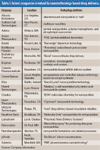
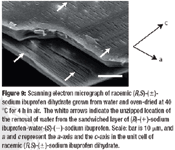
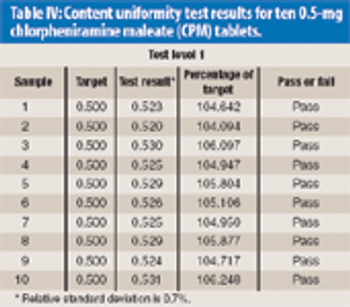
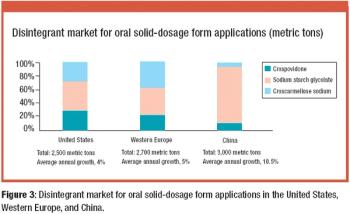
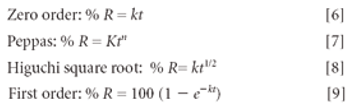The Active Pharmaceutical Ingredients Committee (APIC) - a sector group of Conseil European des Federations de l'Industrie Chimique (CEFIC) - first voiced the need for EU GMP API legislation in 1993 to help ensure the safety of medicines. In 2000, the International Conference on Harmonisation (ICH) finalized the harmonized API GMP Guideline Q7, which became legal in the US and Japan in 2001. The EU adopted a directive in March 2004 that includes the requirement for APIs in medicines for the EU market to comply with ICH/Q7A. Member States are transposing the directive into their national law: about half of them have completed this process, seven more are well on their way to completion, while seven others are still in earlier stages of adoption.