
Valerius Biopharma will use Catalent’s GPEx technology to produce cell lines for biosimilar drugs.

Valerius Biopharma will use Catalent’s GPEx technology to produce cell lines for biosimilar drugs.

ExcipientFest has been acquired by IPEC and rebranded as Excipient World.
Naturally occurring and engineered albumins are being explored as a tool to enhance the stability of drugs, including biologics, and extend shelf-life.

The use of more targeted therapies is expanding as the public gains access to low-cost genetic testing, and more advanced computer systems are offering data from healthcare systems.

Smaller review divisions will bring experts closer to decision processes and reduce bottlenecks, FDA leaders say.

Pharmaceutical companies should consider what is required for QIDP designation and whether they can use it to extend the protection afforded to their innovative products.

Choice of carrier can have a significant impact on the capsule filling process as well as the performance of the DPI formulation.

Determining the right process conditions for a freeze-drying cycle requires an understanding of the effect of each step on the drug product.

A skilled workforce is needed to deliver on technology’s promising medical advances.

Bioprocess understanding, the right equipment, and automation help, but multifunctional teamwork is the key to API production success.

Proteus Digital Health is collaborating on a pipeline of digital, oral solid-dosage drugs for various therapies, including cardiovascular and oncology drugs, based on its first NDA, which used ingestible sensors in an antipsychotic treatment.

The company will add a new $5-million drug development center of excellence at its facility and headquarters in Somerset, NJ.

Scientists at Washington University School of Medicine in St. Louis have developed a new method that could help increase the long-term effectiveness of gene therapy.

Research from Gladstone Institutes suggests that transplanting genetically altered interneurons could improve cognitive function for Alzheimer’s disease.

Porton will use biocatalyst technologies from Codexis to accelerate API development for clients.
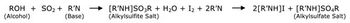
Understanding the advantages and suitability of different methods to measure residual moisture content in lyophilized materials-and the respective limitations-aids in selecting the most appropriate method for testing.

The new platform is expected to speed up cell line development.

LNC Therapeutics has appointed a new CEO and is strengthening its R&D for gut microbiome-based drugs.

The contract renewal allows AMRI to continue to provide medicinal chemistry and ADME services for advancing drug discovery programs in neurotherapeutics.

Johnson & Johnson’s Janssen partners with Bristol-Myers Squibb to advance next-generation therapies for cardiovascular diseases.

The agency has granted breakthrough therapy designation to Roche’s hemophilia therapy for treating hemophilia A in patients without factor VIII inhibitors.

The company is launching a new pre-fabricated, ready-to-run manufacturing facility that is expected to significantly decrease the production timeline for viral vector-based therapeutics.

Sartorius Stedim Biotech will supply equipment for Penn State’s new Fermentation Facility in the university’s Center of Excellence in Industrial Biotechnology.

Under an Innovate UK grant for a three-year project, Arcinova and the University of Nottingham will develop a continuous, flexible modular manufacturing technology platform.

A new report gives an overview of the work of the International API Inspection Program.