
On Wednesday, Oct. 9, 2018, Dr. Caroline Bauer will discuss selecting technologies that enable the progression of compounds that require bioavailability enhancement to achieve target absorption at CPhI Worldwide.


On Wednesday, Oct. 9, 2018, Dr. Caroline Bauer will discuss selecting technologies that enable the progression of compounds that require bioavailability enhancement to achieve target absorption at CPhI Worldwide.

Researchers from Ruhr-Universität Bochum in Germany and the National Institutes of Health modified the protein Nurr1 to enter cells from the outside, possibly enabling the protein to become a drug development candidate for illnesses such as Parkinson’s disease.

Researchers at Vanderbilt University Medical Center have isolated the first human monoclonal antibodies (mAbs) that can neutralize norovirus, a virus that causes acute gastrointestinal (GI) illness.

Coperion’s ZSK 18 MEGAlab extruder fulfills requirements for both wet extrusion and hot-melt extrusion pharmaceutical processes.

Advances in medicine and consumer electronics can enhance drug delivery and patient care.

The collaboration will explore the potential of Dyadic’s gene-expression platform to produce multiple biologic vaccines and drugs.

As it investigates the root cause of an impurity discovered in valsartan, FDA extends its studies to APIs with similar synthesis processes.

The European Commission’s proposed amendment on SPC waivers has sparked opposing views from drug originators and producers of generic drugs and biosimilars.

Sharing know-how can help resolve common bio/pharma technical challenges.

Existing software tools cannot take into account the complexity of disease.

Increasingly complex trial protocols have added to IMP manufacturing challenges.
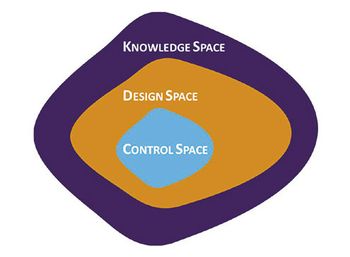
Using a QbD approach in the development and formulation of topical products will enable the drug developer to provide a robust control strategy for manufacturing.
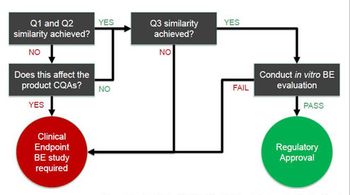
This article examines IVBE testing requirements for topical creams and explores some of the analytical techniques necessary.

The human skin protects the body from physical, mechanical, and chemical insults while preventing endogenous water loss. This function is predominantly achieved by a thin (10–30 µm) cornified outermost layer-the stratum corneum (SC)-generated through terminal differentiation of the basal epidermal keratinocytes. The stratum corneum protects the human body, but also severely limits drug delivery into and across the skin.

Thixotropic gels, thermosoftened systems, and self-emulsifying systems have expanded the range of potential excipients for liquid-filled hard capsules (LFHC).

API-in-capsule approaches enable pharmaceutical companies to quickly assess new drug candidates with reduced API consumption and to increase speed to clinic.

Protagen Protein Services, a CRO, now offers quicker and more accurate characterization of biomolecular stability using differential scanning calorimetry (DSC).

The new Testa Center in Uppsala, Sweden is a collaborative test bed offering biotechnology equipment from GE Healthcare for process development.

Researchers from the Department of Chemistry and Warwick Medical School developed a way to synthesize polymers to accelerate antimicrobial activity screening.

Pharmaceutical companies are developing new strategies to address the ever-increasing development costs for new drug therapies and maximize the value of their existing drug portfolio.

The agency issued a draft guidance on developing new medication-assisted treatments for opioid-use disorder.

The companies will collaborate on the discovery, development, and commercialization of cell therapies for cancer.

Boehringer Ingelheim joins Oxford BioMedica, UK Cystic Fibrosis Gene Therapy Consortium, and Imperial Innovations to form a partnership for developing a new gene therapy to treat cystic fibrosis.

This article describes the approaches used during the development of a dexlansoprazole delayed-release orally disintegrating tablet (ODT) to evaluate tablet size and texture as they relate to disintegration rate and patient experience; in addition, the resistance to alcohol was also characterized.

Low-temperature chemistry enables performance of more challenging and selective chemistry.