
Collaboration and single-use technologies aided the rapid scale-up of Ebola vaccine manufacturing

Collaboration and single-use technologies aided the rapid scale-up of Ebola vaccine manufacturing

AMRI’s acquisition of Euticals expands its API development and manufacturing business.
The production of antibody-drug conjugates requires biopharmaceutical and chemical manufacturing, and conjugation capabilities.
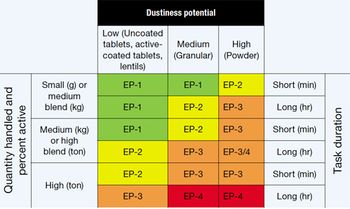
Safe handling of HPAPIs requires determining exposure potential and selecting appro-priate containment strategies.

Bioprocess operations-from cell line selection to final filtration-can influence the consistency and purity of biologic drug substances.

Troubleshooting and collaboration are essential in implementing commercial lyophilization processes.

Application for Sirdupla Uniformity of Delivered Dose Methodology

Armin Gerhardt, associate professor of Pharmaceutical Science, Concordia University Wisconsin School of Pharmacy, discusses the effects of moisture on product quality and how to achieve good control of moisture during pharmaceutical manufacturing operations.

The author looks at EUCAST’s role in tackling the spread of antimicrobial resistance, by creating definitions for a global consensus.

Immuno-oncology drugs are demonstrating patient benefits, but growing resistance to the high cost has implications for patients, market access, and manufacturers.

The agency publishes draft guidance on assay development and validation for immunogenicity testing.

Shire announces plans to build a flexible biopharmaceutical manufacturing facility in County Meath, Ireland.

FDA explains the rewards that may be associated with switching from batch to continuous manufacturing.

FDA approved an update in the manufacturing of Prezista (darunavir) using a continuous manufacturing line at Janssen Supply Chain’s facility in Puerto Rico.

Cornell researchers reveal that an existing FDA-approved drug can facilitate the delivery of other large molecules across the blood-brain barrier.

Kollicoat MAE 100-55 from BASF can be used as a direct substitute in commercial enteric pharmaceutical formulations.

Transferring the manufacturing of a drug from one scale to another or between manufacturing sites presents both technical and business challenges.

Pharmaceutical Technology spoke with Bill Randolph, vice-president, Technical Services, Janssen Supply Chain, about some of the considerations for technology transfer of a continuous, solid-dosage manufacturing process and what he sees as the outlook for continuous manufacturing.

Manufacturing highly toxic compounds in a biopharmaceutical environment tests equipment and systems.

A global API marketplace increases the burden of supply chain monitoring for drug companies.
Choosing the right container and container closure system is crucial for ensuring product quality, safety, and efficacy of a biologic formulation.

Headspace moisture analysis is a rapid non-destructive analytical method that may potentially address the limitations of traditional methods used for residual moisture determination.
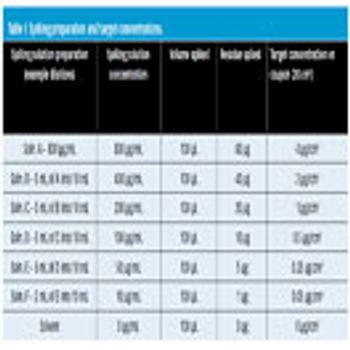
Visible residue limits have been shown to be a valuable tool in validated cleaning validation program.

Unsafe material may remain in the US supply chain, according to a March 29th letter to FDA Commissioner Califf

Novo Nordisk broke ground on a facility in Clayton, NC, to manufacture APIs for GLP-1 and insulin medicines.