
The authors present analysis of the state of control of intermediate identity and quality, based on analysis of recently submitted DMFs.

The authors present analysis of the state of control of intermediate identity and quality, based on analysis of recently submitted DMFs.

This article explores two commercial platforms, and touches on a new program underway in the UK.

Stephen Hoag, a professor at the University of Maryland (Baltimore), and a member of the National Institute of Pharmaceutical Technology and Education (NIPTE) offers a brief update on issues, and NIPTE’s database project.

Matt Richardson of Capsugel and Michael Morgen of Bend Research, discuss improvements and results that have been seen in the second generation of hydroxypropyl methyl cellulose (HPMC) materials.

Hovione will operate a commercial-scale continuous manufacturing facility in New Jersey as part of an agreement with Vertex Pharmaceuticals.
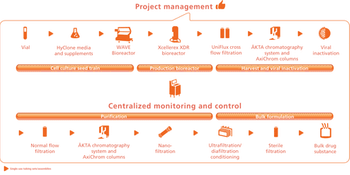
Asking the right questions is crucial to establishing a biopharmaceutical facility design.

Gauging the adequate level and type of screening is the challenge when identifying relevant crystalline forms.

Analytical laboratory equipment adapts for new realities of downsizing, outsourcing, and speed demands.

Hazardous reagents can prove to be faster, simpler, cheaper, and greener.

Choosing the right facility size requires tailoring the design for current needs as well as anticipating the future.

As specialty API outsourcing grows, manufacturers and contract development and manufacturing organizations are investing for the long haul.

The pharma company revealed in a fourth quarter call that it will improve its cell-culture capabilities by focusing on the use of naïve, highly proliferative cells to manufacture its CAR-T drug candidate.

The author lists five key areas to consider when selecting a CDMO to develop highly potent formulations.

ICIG’s CordenPharma Group expands fermentation-based production technology with acquisition of Sandoz Site.

Ashland Chemical’s Calvert City, KY, facility is the first in the US to receive EXCiPACT certification for GMPs of pharmaceutical excipients.

ViaCyte and Janssen Biotech have entered Phase I/Phase II clinical trials for VC-01, a candidate treatment for the treatment of type 1 diabetes.

The author reviews FDA's final Animal Rule guidance.

Scale-down modeling is instrumental in supporting the development of downstream biopharma manufacturing processes.

Hazardous reagents can simplify processes and provide higher yields and purities.

The chemical distribution industry has formed an international chemical trade association to address global issues.

Choosing the right excipient manufacturer can help ensure the use of quality excipients.

Sustainable harvesting combined with CMO expertise helped Centroflora CMS ensure supply continuity after it acquired Boehringer Ingelheim's non-captive API phytochemicals portfolio.

Steven Denham, director of biostatistics at MPI Research, discusses the impact the Standard for Exchange of Nonclinical Data may have on the pharmaceutical industry.

For successful partnerships, it’s important to take a long-term view, focus on simple designs, and address potential payer concerns up front.