
A study backed by the Centre of Regenerative Medicine found that laminins are crucial cell components that could help aid the commercial production of stem cell therapies.

A study backed by the Centre of Regenerative Medicine found that laminins are crucial cell components that could help aid the commercial production of stem cell therapies.

Ash Stevens has received FDA’s approval to manufacture Takeda’s multiple myeloma drug, ixazomib, at its facility in Riverview, Michigan.

Operations at Catalent’s Beinheim, France, softgel facility were suspended following suspected deliberate action to misplace capsules.

BioOutsource releases informational video detailing issues associated with ADCC assays and how to effectively analyze them.

Almac is investing GBP16 million to expand its formulation and analytical development services and has successfully negotiated to operate in the Charnwood Campus in Loughborough, England.

BASF’s new multi-product amines plant in Ludwigshafen, Germany meets growing demand for amines in various applications, including pharmaceuticals.

Efficient syntheses are possible using multi-component and cross-dehydrogenative, heteroaromatic C–H silylation reactions

The European AIDS Vaccine Initiative has brought together leading HIV researchers from public organizations and biotech companies to develop protective and therapeutic HIV vaccines.

The Transform hydrate-able film from Lubrizol LifeSciences is compatible with a range of APIs.

Ashland presented a science-based strategy to enhance pharmaceutical formulation quality and advance drug delivery at AAPS 2015.
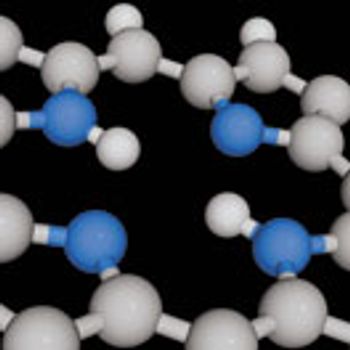
Efficient syntheses are possible using multi-component and cross-dehydrogenative, heteroaromatic C-H silylation reactions.

FDA’s inactive ingredients database (IID) has been under development for several years, as a way to allow generic and name-brand pharmaceutical manufacturers to check on the safety and performance of specific inactive ingredients.

Croda Chocques SAS' excipient facility in France has received EXCiPACT certification from SGS ICS, making it the 24th manufacturing site to be certified to the scheme, which verifies that manufacturing of pharmaceutical-grade excipients meets current good manufacturing practices (cGMPs). It is the third Croda site in Europe to receive this certification in the past year. Certification reflects a rigorous assessment program, both for the auditor and the audited, and the auditor's report had to be verified by an independent certification board.

Catalent’s SMARTag™ technology enables biologic innovators to develop more efficacious antibody drug conjugates. It allows site-specific, programmable drug placement using proprietary cytotoxin-linkers/conjugation chemistry in an efficient and scalable process.

The authors look at key factors driving the risk-sharing agreements that have been implemented to reduce drug expenditures across Europe.

Dow introduces solvent-free productivity technology for coating barrier membranes.

EMD Millipore, the life-science division of Merck KGaA, has introduced Parteck SRP 80, a new functional excipient for oral sustained-release formulations. The excipient is polyvinyl alcohol (PVA)-based and fully synthetic-according to EMD Millipore, this feature ensures batch-to-batch and performance consistency and facilitates quality by design (QbD) and validation processes.

Pharmaceutical and biopharmaceutical manufacturers must start thinking about new ways to navigate the evolving healthcare marketplace while continuing to deliver life-changing therapies to patients and responding to the demands of an increasingly informed and sophisticated customer base.

The excipient supplier's cGMP practices have been certified as meeting EXCiPACT standards

Athenex will build two facilities for high-potency, oncology API ingredients in Chongqing, China.

The FDA grants to the Rutgers-led C-SOPS research consortium will support the introduction of continuous manufacturing techniques for pharmaceuticals.

Hovione is investing in specialized formulation capabilities, beginning with the acquisition of a formulation facility adjacent to the current process chemistry and particle engineering facility in Loures, Portugal, the company announced on Oct. 19, 2015. This acquisition is a strategic investment to further boost development and manufacturing capabilities for both inhalation and oral dosage forms.

At CPhI Worldwide 2015, The Dow Chemical Company announced the global commercial availability of AFFINISOL HPMC HME, a new generation of cellulosic polymer for drug solubilization. The polymer is designed for use by pharmaceutical companies looking to enhance the solubilization and inhibit the recrystallization of APIs in hot-melt extrusion (HME) formulations.

The new sugars are touted to increase the efficacy of active pharmaceutical ingredients and improve cell culture yield.

Umicore is one of the largest producers of platinum APIs for anti-cancer treatment. We pride ourselves in having decades of experience in platinum group metals chemistry and high potency APIs.