
Cambrex Corporation has announced facility, equipment, and instrument upgrades at its High Point, NC facility and updated progress on expansions in Cedar City, IA.

Cambrex Corporation has announced facility, equipment, and instrument upgrades at its High Point, NC facility and updated progress on expansions in Cedar City, IA.

The company is increasing its cell culture media production capacity at its facilities in Pasching, Austria, and Logan, Utah.

Valerius Biopharma will use Catalent’s GPEx technology to produce cell lines for biosimilar drugs.

Choosing the right contamination control platform requires considerable research into what a product needs for an effective process design.

Understanding the advantages and suitability of different methods to measure residual moisture content in lyophilized materials--and the respective limitations--aids in selecting the most appropriate method for testing.
Naturally occurring and engineered albumins are being explored as a tool to enhance the stability of drugs, including biologics, and extend shelf-life.

Barriers impede biosimilar market entry into the United States despite the Biologics Price Competition and Innovation Act.

The technology, known as Verifi, is available with the ChargePoint’s range of valves that ensure safe and contamination-free handling of API and other formulation ingredients.

The companies expanded their partnership to develop and commercialize messenger RNA (mRNA) cancer vaccines to include shared-antigen mRNA cancer vaccines such as mRNA-5671.

The company received a complete response letter from FDA in response to the biologics license application for a proposed rituximab biosimilar.

The data and analytics company reports on the anticipated uptake of Humira (adalimumab) biosimilars in the EU once they are launched in 2018.

Lonza announces addition of mid-scale biologics manufacturing capacity and cell-therapy suites at Portsmouth, NH site.

Choice of carrier can have a significant impact on the capsule filling process as well as the performance of the DPI formulation.

Public health authorities and the biomedical research community are seeking new strategies to address global health threats.

A skilled workforce is needed to deliver on technology’s promising medical advances.

Bioprocess understanding, the right equipment, and automation help, but multifunctional teamwork is the key to API production success.

The agency updated its list of recommended influenza virus strains that manufacturers should include in vaccines for the autumn 2018 season.

After a review of public comments, USP will not move forward with nomenclature proposal without further FDA collaboration.

The company received a complete response letter from FDA in response to the biologics license application for a proposed trastuzumab biosimilar.

Proteus Digital Health is collaborating on a pipeline of digital, oral solid-dosage drugs for various therapies, including cardiovascular and oncology drugs, based on its first NDA, which used ingestible sensors in an antipsychotic treatment.
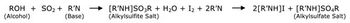
Understanding the advantages and suitability of different methods to measure residual moisture content in lyophilized materials-and the respective limitations-aids in selecting the most appropriate method for testing.

The new platform is expected to speed up cell line development.

Johnson & Johnson’s Janssen partners with Bristol-Myers Squibb to advance next-generation therapies for cardiovascular diseases.

The agency has granted breakthrough therapy designation to Roche’s hemophilia therapy for treating hemophilia A in patients without factor VIII inhibitors.

Sartorius Stedim Biotech will supply equipment for Penn State’s new Fermentation Facility in the university’s Center of Excellence in Industrial Biotechnology.