
Eppendorf’s Conical screw cap tubes in 15 mL and 50 mL complete a range of volume sizes that meet needs from 0.5 mL to 50 mL.

Eppendorf’s Conical screw cap tubes in 15 mL and 50 mL complete a range of volume sizes that meet needs from 0.5 mL to 50 mL.

Will biosimilars share a compendial identity like generic drugs do?
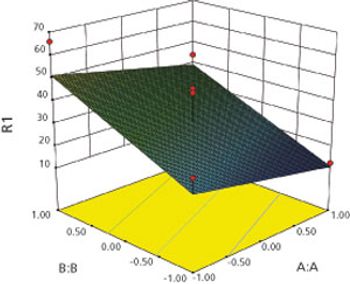
Box-Behnken modeling was used to optimize a resinate complex, to mask the taste of levocetirizine dihydrochloride and montelukast sodium in orally disintegrating tablets.

Gyrolab CHO-HCP Kit 1 detects and quantifies host cell proteins from Chinese hamster ovary cells.

The draft guidance document clarifies chemistry, manufacturing, and controls information for marketing applications.

Experts attending the European Psychiatry Association Congress in Vienna say that Adasuve has made an impact in the treatment of agitation in patients suffering from schizophrenia or biopolar I disorder.

Pharmaceutical companies are constantly aiming for shorter drug-development cycles and advances in formulation development produce significant benefits.

This new pharmaceutical stability storage facility will enable the company to expand its analytical and formulation offerings to the pharmaceutical and biotech industries.

Widespread use and abuse of opioid painkillers is prompting efforts to develop new drugs and formulations that resist abuse while providing relief to legitimate patients.

Eight physician groups wrote to the commissioner of FDA to stress the importance of transparency in biosimilar labeling to decrease prescribing risks.

As the biopharma industry awaits FDA’s guidance on biosimilar naming, brand and generic manufacturers establish positions.

Medicago's new production facility will make plant-based vaccines and therapeutics.

The new guideline replaces the previous version-CHMP/EWP/240/95 Revision 1.

Alexion will construct a biologics facility in Ireland that is its first outside the US.

This study evaluates the impact of controlled nucleation on the ability to optimize a lyophilization cycle for a monoclonal antibody formulation.

Pall has agreed to be acquired by Danaher for $127.20 per share.

With acquisition of Oncaspar portfolio for leukemia from Sigma-Tau Finanziaria, Baxter BioScience adds an oncology infrastructure and biologic.

The agency publishes draft guidance answering industry questions about the Biologics Price Competition and Innovation Act.

A vaccine patch may eliminate the need for traditional means of vaccine distribution, according to an article in NPR.

The US Court of Appeals granted Amgen’s request to block Novartis’ Neupogen biosimilar, Zarxio, from the US market until the court resolves litigation between the two companies.

Eleven of the leading US biosimilar developers have collaborated to form the Biosimilars Forum, a nonprofit organization formed to expand patient access to biosimilars.
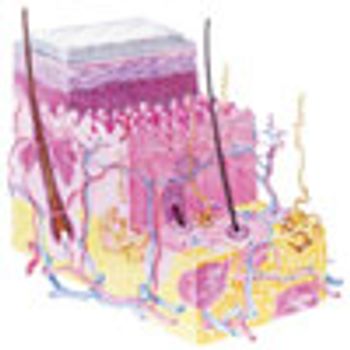
Advances in transdermal drug delivery, particularly with microneedles, are enabling a wider range of drugs to be delivered through the skin.

Intellectual property lawyers estimate biosimilar litigation will swell as early as 2018.

While the United States and Europe still dominate, CMOs and CROs based in emerging markets continue to capture market share.

An integrated pilot plant tests heteronucleation and continuous crystallization.