
Modern methods and modeling offer a better way to understand solubility issues and solve today’s complex formulation challenges.

Modern methods and modeling offer a better way to understand solubility issues and solve today’s complex formulation challenges.
Prodrugs and drug-delivery systems controlled by time, pH, and osmosis, are being used to prevent drug degradation in the stomach and large intestine and ensure drug release in the colon.

Anton Paar’s MCP polarimeters are now designed with a new LED light source and optional integrated air pump to clean and dry sample cells.
Delivery systems that allow drugs to be administered as liquids, but to form gel within the eye, promise to improve efficacy and patient compliance.

The addition of a new manufacturing line at Lonza’s Portsmouth, NH site enables Alexion to add dedicated product supply for 10 years.

The new facility expands the company’s commercial manufacturing capability at its Bend, Ore. site.

Eric Langer, Managing partner at BioPlan Associates, speaks with Pharmaceutical Technology about the results from the 12th annual BioPlan Associates survey, and single use technology adoption.

Eric Langer, Managing partner at BioPlan Associates, speaks with Pharmaceutical Technology about biosimilar development trends.

Brady Cole, VP of Commercial Operations, at ABEC, spoke with Pharmaceutical Technology about biopharmaceutical manufacturing processes.

Barry Holtz, Principal, at Holtz Biopharma Consulting, and Klyo Collaborative, spoke with Pharmaceutical Technology about collaborative success strategies for biopharm companies.

The new column features Natrix’s signature macroporous hydrogel.
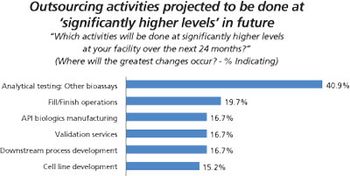
More biopharma companies choose outside service providers for assay testing.

The divestment of Nimenrix and Mencevax was to satisfy regulatory clearances when GSK gained Novartis’ vaccines business in an asset swap in March 2015.

Juniper Pharma Services has entered into a long-term collaboration with OxSonics to support the development, scale-up, and GMP manufacturing of OxSonics’ proprietary sono-sensitive particles.

The agency clarifies its requirements for allowable excess volume and labeled vial fill size in injectables and biologics.

Reach Separations has announced plans to double its laboratory space at its Nottingham facility as a result of increased demand for its specialist purification services.

Monoclonal antibodies can facilitate the entry of radiopharmaceuticals into cells, David Scheinberg said at BIO 2015.

The agency takes action against websites that illegally sell unapproved medications.

Biomax Informatics AG and Pierre Fabre announced that they have entered into a collaboration to create an integrated antibody sequence and knowledge database.

Experts at Eppendorf discuss common challenges in cell culture and share insights on possible solutions.

BPTF seeks changes in performance goals and fee payment schedule in GDUFA renegotiations.

The product, marketed as Sirdupla in the United Kingdom, is the generic version of GSK’s beta agonist and corticosteroid combination treatment for asthma.

Designed with safety standards in mind, flowtherm Ex incorporates a number of connectable sensors, such as vane wheel, vortex, thermal, Pt100, and other physical sensor with analog output, thereby allowing a range of applications.

Bosch’s market launches at the show include a new bioreactor for laboratory-scale API development, a water-for-injection unit, and a new generation of pure steam generators with high yields.

Products on display include the newest addition to the Mobius line of single-use bioreactors; Cellvento CHO cell culture media for optimized production of specific cell lines; and new high-volume AFS water purification systems.