
The European Chemicals Agency (ECHA), the European Union regulatory body overseeing the implementation of the REACH (Registration, Evaluation, and Restriction of Chemicals) regulation, began full operations on June 1.

The European Chemicals Agency (ECHA), the European Union regulatory body overseeing the implementation of the REACH (Registration, Evaluation, and Restriction of Chemicals) regulation, began full operations on June 1.

In an assessment report about medicinal products containing heparin, the European Medicines Agency (EMEA)'s Committee for Medicinal Products for Human Use (CHMP) said it could not draw firm conclusions about the level of risk associated with unfractionated heparins (UFH) contaminated with oversulfated chondroitin sulfate (OSCS). Nevertheless, CHMP recommended that contaminated lots be withdrawn completely.

Also, Shire voluntarily recalls ADHD patch Daytrana

Also, Pall plans expansion in South America, Anthony Clarke joins Alexza Pharmaceuticals, more...

The global and US biotechnology industries performed well in 2007 as each recorded higher revenues and inched toward aggregate profitability.

As part of a larger effort to address drug counterfeiting, the European Commission is seeking to tighten manufacturing and related supply-chain requirements for active pharmaceutical ingredients.

Antibody drug conjugates offer a niche opportunity in drug development and contract manufacturing.

Nanoparticle-based systems present many advantages for the delivery of current and emerging biological drugs.

The good, the bad, and the ugly about direct-to-consumer advertising.
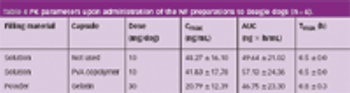
When drugs are encapsulated, electrification (the electrostatic charge of the capsule) may sometimes cause problems, such as capsule adhesion during transportation or dispersion of the capsule content in the filling process.

The US Senate approved a measure (HR 2642) that would provide the US Food and Drug Administration with $275 million in additional funding under a supplemental appropriations bill. The measure now goes before the House.

Also, GVK BIO and Wyeth Pharmaceuticals form research agreement, Eli Lilly announces changes to management, more...

The European Federation of Pharmaceutical Industries and Associations (EFPIA), the trade association representing European pharmaceutical manufacturers, issued recommendations to the public consultation launched in March by the European Commission's proposed drug anticounterfeiting measures. EFPIA's proposal includes a ban on drug repackaging.

Also, Pfizer to close Indiana "Exubera" facility, executive appointments at Patheon, more...

Also, Quintiles Transnational to acquire Eidetics, ChemAxon appoints Alex Drijver CEO, more...

Eli Lilly completed the final and largest phase of a major biotechnology expansion in Indianapolis, Indiana.

Increased investment by the pharmaceutical and biotechnology majors in offshore early drug development creates opportunities and challenges for outsourcing.

Congress is considering proposing a new chemical policy similar to the European Union’s REACH (Registration, Evaluation, Authorization, and Restriction of Chemicals) regulation. Batch and custom manufacturers weigh in on the debate.

Congressional hearings were held last week on the Food and Drug Administration Globalization Act discussion draft.

Also, FDA removes OAI status for Watson's Florida facility, executive management changes as GSK, more...
Scientists are giving up on a preventive vaccine for AIDS, but there are lessons to be learned.

Florida is making its case for pharmaceutical and biotechnology research and development.

The less complex nature of excipient manufacturers, as compared with API manufactures, carries many benefits.
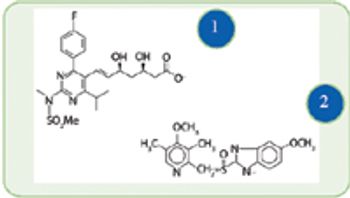
Chemocatalytic and biocatalytic routes show promise for more efficient syntheses of select active ingredients.
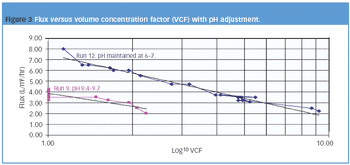
The pharmaceutical industry must address the release of nonbiodegradable APIs into the environment.