Drug Development
Latest News
Latest Videos

More News

The collaboration will initially focus on advanced stages of solid cancers such as colorectal, pancreatic, lung, and breast, but may expand to other cancer types in the future.

New drug delivery systems prioritize convenience and customization, using advanced technologies like nano-engineering and non-invasive routes to improve patient outcomes.

Increasing demand, complexity, and potency are driving innovation and investment in HPAPIs.

The articles in this issue reflect an industry in transition, committed to innovation while ensuring safe, reliable, and forward-looking pharmaceutical production.

Though not an actual creature, the aptly named longevity fund has announced a strategic investment in Etheros’ development of a new class of drugs.

Large-language models are excellent for general-use AI systems, but they don’t understand pharmaceutical companies’ proprietary documentation—the validated procedures and quality protocols that ensure drug safety. Smaller, domain-specific language models give companies more control and efficiency in their AI use.

FDA has granted orphan drug designation to FS2 (kynurenic acid) for the investigational treatment of idiopathic pulmonary fibrosis.
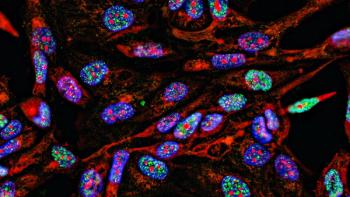
Live cell phenotypic screening can enhance translational predictability and speed up lead optimization.

The first patient visit has taken place for the SURMOUNT-REAL UK clinical trial of Eli Lilly and Company’s weight-loss drug, tirzepatide.

Machine learning tools are now moving from concept to validated applications, driving transformative shifts in bioanalysis, preclinical testing, and formulation optimization across the industry.

The conclusion of this interview with Sanjay Konagurthu focuses on simulations and calculations that go beyond simple screening.

The European Union aims to become the most attractive destination for clinical research.

Corey Bloom of Lonza outlines major points from his AAPS presentation, from drug delivery technology selection to the importance of CDMO partnerships.

Take our survey to voice your opinions on the bio/pharma industry impacts of the FDA Commissioner’s National Priority Vouchers program.

PharmSci 360 will spotlight the economics behind AI, scale-up, and advanced modalities, addressing cost barriers that influence future drug manufacturing.

Pharmaceutical Technology® spoke with Dr. Mark Arnold, owner and principal, Bioanalytical Solution Integration, ahead of AAPS PharmSci 360 to find out how bioanalysis enhances bio/pharmaceutical drug development.
Radiopharmaceuticals are allowing for the targeted treatment of cancers, but they do pose challenges.

Pharmaceutical Technology® spoke with Tara Dougal, Event Director for Pharma at Informa Markets about what attendees at CPHI Frankfurt should expect this year.

Real-world data can be utilized to ensure quality and effectiveness of drug products.

Non-parenteral alternatives for biologics remain a clinical imperative and a formidable challenge.

Recent developments show how innovations and treatments for cancer are rapidly advancing.

The first needle-free adrenaline treatment launches in the UK, validating non-invasive delivery with real-world data comparable to injection effectiveness.

Take this quiz to test your comprehension of one of our recent feature articles.


Michael Ritchie, chief commercial officer at Champions Oncology, explains what makes radiopharmaceuticals unique in the treatment of cancer.