
Researchers recently developed a drug-delivery system to mitigate problems associated with jet-injection drug delivery, and also improved on the design and operation of microscale actuators as a possible drug-delivery method.

Researchers recently developed a drug-delivery system to mitigate problems associated with jet-injection drug delivery, and also improved on the design and operation of microscale actuators as a possible drug-delivery method.

Catalent Pharma Solutions, a provider of drug and biologic development services, delivery technologies and supply solutions, has officially opened a new biomanufacturing center of excellence in Madison. The facility, which was constructed in response to customer demand, is expected to quadruple Catalent's current biologics manufacturing capacity and extensively utilise single-use technology for greater flexibility and efficiency. It will allow the company to extend its offerings in the biologics sector while enhancing the efficiency and output of its proprietary GPEx cell line engineering technology as well as other mammalian cell lines.

VAI?s Sterile Cleanroom Wipers use innovative manufacturing technologies and a single source, dedicated supply chain allows for traceablility.

FDA will use a new anticounterfeiting tool to detect fake medicines.

Increasing production efficiency while ensuring high parenteral product quality is the goal of both manual and automated visual inspection systems.

BioOutsource, a contract testing services provider to the biopharmaceutical industry, has opened a new facility in Cambridge, Massachusetts. The facility marks the company's first North American offices, which were opened in response to increasing demand for the company's bioanalytical and biosafety services, particularly in the field of biologic and biosimilar characterisation.

EMA has upgraded its EudraGMP database to include information on GDP in addition to GMP. The new EudraGMDP database is a key deliverable of the Falsified Medicines Directive (FMD) that came into effect in January 2013. The aim is to increase supply chain security in the EU by making supervision of manufacturing and distribution of medicines more robust to ensure supplier compliance.

PIERRE FABRE MEDICAMENT PRODUCTION has 22 years experience in isolator technology for aseptic filling of high potent freeze-dried injectable products.

Shimadzu's LCMS-8040 combines newly improved ion optics and collision cell technology with proprietary ultrafast technologies.

An annual Healthcare Compliance Packaging Council competition recognizes innovative pharmaceutical packages designed to improve patient adherence.

Speakers discuss modularization, single-use systems, and process validation in a series of podcasts available on the Pharmaceutical Technology website.
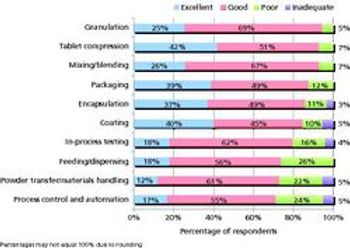
Pharmaceutical Technology's survey of manufacturing equipment and trends shows satisfaction with utility and innovation in most solid-dosage and parenteral drug-manufacturing equipment.


Mary Storch, a member of the planning PDA supply chain committee, is associate director, external QA oversight, for Ben Venue Laboratories. She discusses parenteral drug-supply chain challenges, in terms of the complexity of the raw materials supplied as well as additional consideration in end-product distribution, such as cold-chain requirements.

As the strategic value of emerging markets increase, pharmaceutical companies increase their R&D and manufacturing investments.

As an important method for improving the stability of parenterals, lyophilization is fairly well understood, but can still benefit from several advancements in the technology.

With high productivity achieved, makers of cell-culture media are working to optimize product quality through better understanding and control of raw materials and production processes.

The trick to taste-masking in solid dosage forms is to never let the taste buds have a chance.

Teva and Lonza have announced that their joint venture will continue to develop, manufacture and market affordable, efficacious and safe biosimilars.

Eisai and Biogen Idec pursue an innovative approach to capacity management.

A Pharmaceutical Technology survey shows satisfaction with utility and innovation in most solid dosage and parenteral drug-manufacturing equipment.

The annual INTERPHEX show presents end-to-end packaging solutions.

Industry is moving toward closed-loop control of continuous processing.

A Q&A with Michael Lacey of the National Institute for Bioprocessing Research and Training
Experts describe best practices for sterility assurance in parenteral drug manufacturing. This article contains bonus online-exclusive material.