
Russell Madsen, group leader of the Parenteral Drug Association (PDA) Filtration Interest Group, discusses technical and regulatory considerations in filtration for parenteral drug manufacturing

Russell Madsen, group leader of the Parenteral Drug Association (PDA) Filtration Interest Group, discusses technical and regulatory considerations in filtration for parenteral drug manufacturing

Equipment suppliers are helping the pharmaceutical industry move towards adoption of continuous tablet production.

Loss-in-weight feeders provide high accuracy for feeding powders.

Bristol-Myers Squibb embarks on a multi-year journey to overcome the challenges of serialization and reap the benefits.

Process analytical technology (PAT) is being successfully used to improve understanding and optimize pharmaceutical unit operations, but greater value can be obtained by integrating PAT with overall process control of a continuous manufacturing system. Pharmaceutical Technology spoke with Ivo Backx, manager of business and project development for the pharmaceutical industry at Siemens Industry Automation Division, to gain insight on the issues involved.

Dow Chemical and Bend Research are developing and commercializing new materials for spray-dried dispersions that address technology gaps in manufacturability and delivery.

Matthieu Egloff, product director with ATMI LifeSciences, discusses the need for larger, special reactors that can provide the necessary conditions for the production of fragile cells on a larger scale.

The growth in biologics drugs and vaccines is leading to greater demand for prefilled syringes, which provide greater safety and convenience for healthcare workers and patients.

America's biopharmaceutical companies are using biological processes to develop 907 medicines and vaccines targeting more than 100 diseases, according to a new report.

Roche and BioLamina have entered into a research and development agreement to jointly develop new cell culture systems for various applications, including stem cell research. The collaboration will assess laminin-based in-vitro cell culture matrices that can offer physiological microenvironments for living cells.
As the strategic value of emerging markets increase, pharmaceutical companies increase their R&D and manufacturing investments.

Room-temperature sterilization using nitrogen dioxide gas benefits parenteral drugs.
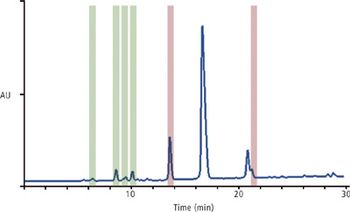
The authors demonstrate that using supercritical fluid chromatography offers distinct advantages in speed and in clean isolation of the desired peaks.

FDA's requirements for API manufacturers in regards to ICH Q7.

Applying quality-by-design and process analytical technology facilitates process understanding and control of various operations in lyophilization.

QbD paradigm advances process understanding in development and manufacturing.

New product reviews for March 2013, featuring automation, IT, and process control systems.

Vaccine development is benefiting from manufacturing advances and support for global health.

The authors review developments in wet granulation using a twin-screw extruder.

Recent advances in equipment design and operation in spraying, drying, and mixing can improve the tablet-coating process.

The tableting science anti-research (TSAR) project seeks to understand why certain formulations stick to tablet tooling.
A roundtable discussion of the challenges and innovations in tablet splitting featuring Freeman Technology, Accu-Break Pharmaceuticals, and Medelpharm.

An alternative chapter has been added to the European Pharmacopoeia for dosage uniformity.

Equipment purchased today for the packaging line should be serialization-ready in preparation for upcoming requirements.

A Q&A with Michael Lacey, plant manager at the National Institute for Bioprocessing Research and Training.