
Medication safety and efficacy depend on maintaining products at the proper temperature.

Medication safety and efficacy depend on maintaining products at the proper temperature.
The share of biologic-based drugs in the global pharmaceutical market is on the rise.

Quality assurance of biological products is central to India's good distribution practices guidelines.

Measuring the rouge corrosion rate can help determine when a system should be cleaned so the final product is not impacted

A thorough investigation of all possible causes of deviations should be performed.

Eastern Europe is moving towards a goal of harmonized regulations.

The minimum amount of residue that can be visually detected is demonstrated for a small number of active pharmaceutical ingredients (APIs) on a range of different surface materials.

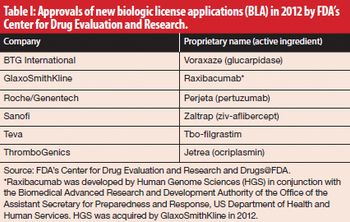
New product reviews for February 2013.
Adopting a seven-step process to maintenance and storage improves tableting quality.

Protecting patients from counterfeit medicines is a pressing issue facing governments and the pharmaceutical industry.

Even in an industry in which all product development is complicated by the intricacies of human biology, orally inhaled products (OIP) stand out as singularly demanding.

Recent activity in standards-setting organizations has raised interest in the impact of testing for impurities that may enter the product before it is mined or harvested or even due to intentional use of some reagents.
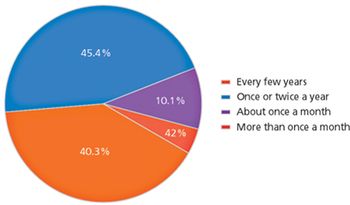
A UC Berkeley survey provides insight into biopharma's risk concerns and strategies.

Innovation resulting in improved productivity continues unabated and is a primary driver for many of the current biopharmaceutical trends.

The ongoing economic crisis in Europe has accelerated healthcare cost-containment measures targeting the price of pharmaceuticals, but the pharma industry is not giving in without a fight.

A new international agreement to reduce mercury contamination of air and water was recently adopted by 140 countries, without a proposal that threatened to limit access to vaccines in much of the world.

FDA has approved Protein Sciences's FluBlok, a seasonal influenza vaccine made with novel technology. FluBlok uses recombinant DNA and a modified baculovirus (a virus that infects insects) to produce a safe and effective human flu vaccine. FDA approved Flublok for people 18–49 years old.

Recently, after reading about the severity of this year’s flu season, I finally went and got my vaccine, which my doctor had been out of when I tried in October.

Adopting a seven-step process resolves tableting problems.

Advances in data loggers and radio-frequency identification tags help meet the increasing need for managing the pharmaceutical cold chain.

Micronization can be performed with a jet mill or bead mill.

The FPC50W is an aseptic filling system with full or partial stoppering and crimp capping of vials, delivering fill volumes ranging 0.1ml to 100ml and filling accuracy of +/- 0.5%.

Janssen Pharmaceuticals, Inc. and GlycoVaxyn AG have entered into a three-year agreement to collaborate on the research and development of a multivalent bacterial vaccine utilizing GlycoVaxyn's bio-conjugation technology.

FDA talks about the changing scope of regulatory science and its effect on drug reviews, site inspections, and overall approaches.
Is process-centered organization in biopharmaceutical manufacturing a stepping stone or a stumbling block?