
Pharmaceutical Technology Europe brings together suppliers of tabletting equipment and excipients to discuss the challenges, innovations and latest trends in the tabletting industry.
The main challenge for tablet manufacturers processing highly potent APIs (HPAPIs) is to protect equipment operators from the inhalation of airborne particles and prevent skin contact with the product during the entire production process: dispensing, granulation, tablet compression, coating and packaging.

Pharmaceutical Technology Europe brings together suppliers of tabletting equipment and excipients to discuss the challenges, innovations and latest trends in the tabletting industry.
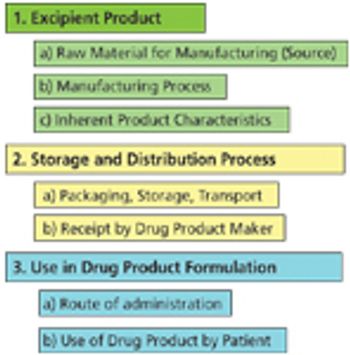
The author describes key considertions for a complete risk-assessment model and provides insight into a pending IPEC guideline in this area.

Nanotechnology often is associated with parenteral drug delivery, particularly for anticancer therapies, but it also has applications in oral drug delivery

The author reviews Warning Letters issued between 2000 and mid-2010 for aseptic processing and non-sterile processing, and determines how many observations were made for each section of the GMPs.
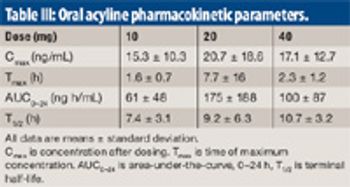
The authors examine an oral-absorption-enhancement technology based on surface-active materials to increase apical membrane fluidity in vitro.

On Mar. 23, 2011, sanofi-aventis offered $7 billion in notes to fund its acquisition of Genzyme. The notes are offered in six tranches scheduled to come due between 2012 and 2021, and the interest rates range from 0.05% to 4%.

The global market for biosimilars is set to grow from $243 million in 2010 to $3.7 billion in 2015, according to research from Datamonitor, an independent industry analyst company.

The National Institutes of Health?s (NIH) definition of embryonic stem (ES) cells poses new challenges for investigators who seek federal research funding.

Novartis has completed the acquisition of a majority stake in Zhejiang Tianyuan Bio-Pharmaceutical Co., Ltd. — one of the largest privately held vaccines companies in China.

Pfizer is considering divesting some of its businesses to maximize their value, according to remarks made by Mikael Dolsten, Pfizer's president of worldwide research and development, at a Barclays Capital investor conference last Thursday.

In a press release on Mar. 21, 2011, the EMA Management Board gave its approval for the current activities of former executive director Thomas Lönngren.

Novartis acquired a majority stake in Zhejiang Tianyuan Bio-Pharmaceutical, one of the largest privately held vaccine companies in China. The acquisition gives Novartis an expanded presence in the Chinese vaccine market and will facilitate the introduction of Novartis's vaccines into the country.

Last Tuesday, the US Senate approved the "America Invents Act," which is intended to reform the nation ’s patent system. If it becomes law, the bill will establish a first-to-file system by defining an invention ’s effective filing date as the actual filing date of the patent or patent application.

Last Thursday, FDA filed a consent decree of permanent injunction against McNeil, a subsidiary of Johnson & Johnson, for failing to comply with current good manufacturing practice requirements. The action prevents McNeil from manufacturing and distributing drugs from its Fort Washington, Pennsylvania, facility until FDA determines that its operations comply with the law.

Many facilities buy compressed gas tanks or evaporate liquid nitrogen to supply processes with dry, high-purity nitrogen. An in-house nitrogen generator, however, provides several significant benefits.

Growing interest in continuous drug manufacturing has brought greater attention to in-line blending, a process that the petroleum and fine-chemicals industries have used for decades.

We are making an emulsion using a high-shear in-line mixer. It?s a 60-gal batch, and it?s taking nearly an hour to get the results we?re looking for. We have tried changing the in-line mixer?s workheads to a two-stage configuration, but it doesn?t affect the mixing time. Where are we going wrong?

PharmTech's monthly newsletter, Equipment and Processing Report, reviews the Editor's Picks for the February 2011 edition from Emerson Process Management and Spirax Sarco.

Japan has suspended the use of two pediatric vaccines made by Pfizer and sanofi following the deaths of four children in three days.

AstraZeneca announced this week that it will discontinue the production of Pulmicort (budesonide) 100 and 200 µg/dose HFA (hydrofluoroalkane) pressurized metered-dose inhaler (pMDI) due to manufacturing issues related to technical aspects of the device, which prevents the ongoing manufacture of the product.

At a conference on preserving national security at the University of Pittsburgh Medical Center last week, FDA Commissioner Margaret Hamburg stressed the importance of medical countermeasures for responding to natural and deliberate threats to public health.

Compressed tablets are the most widely used solid dosage form so they must satisfy a number of physical requirements in terms of hardness, disintegration ability, friability and uniformity.

One-pot processing is a term that includes any technology that combines different unit operations of a pharmaceutical production process into one machine.

The author presents a method to calculate the relationship between supply air volume flow and airborne particle concentrations.

Moisture Activated Dry Granulation (MADG) was developed in response to the difficulties experienced with wet granulation, in terms of endpoint, drying and milling. Wet granulation process endpoint is very sensitive to granulation time and shear. The wet granules need to be dried to a narrow range of moisture contents, which is difficult. The dried granules need to be milled, but the milled granules often have either too many fines or too many coarse particles (or both) - an undesirable bimodal distribution.