Editor's picks of analytical instrumentation products for May 2011.

Editor's picks of analytical instrumentation products for May 2011.

Regulators and standard-setting bodies are re-examining over-the-counter drugs.
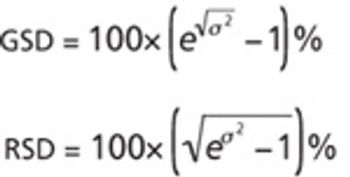
The author argues that traditional concerns about repeatability and intermediate precision remain valid but insufficient.
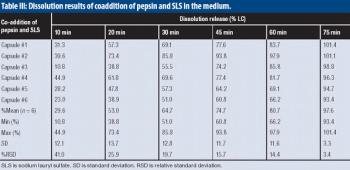
The authors develop a pratical approach to avoid unwanted interactions between pepsin and SLS in dissolution Tier II tests.
The authors examine the influence of glass-transition temperature, melt viscosity, degradation temperature, and process settings.

Emerging methods could provide alternative ways of producing inhalable drug particles.

Approaches in using small-molecule and peptide synthesis offer promise in widening the scope of drug candidates.

Those who doubt there's faith in science, should check out our annual Bioprocessing Survey.

FDA, NIH and industry seek new strategies to spur drug development and promote access to therapies.

Can the semiconductor industry help Big Pharma develop therapies?

Innovator and generic-drug companies need to adapt to compete in the biosimilars market.

Many factors affect research results.

Despite initiatives to encourage multinational pharma companies to conduct R&D in the UK, the country may be losing its edge; is Pfizer's decision to exit a key site earlier this year the beginning of a mass exodus?
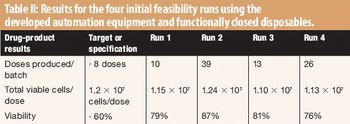
The authors developed automated equipment that uses functionally closed disposables to perform cellular and ribonucleic processing.

The authors discuss various approaches and related issues, including production of difficult-to express proteins using cell-free expression systems, scalability of protein expression, and site-specific chemical modifications.

Traditionally, lot Numbers and expiry codes or dates that appear on cartons have been considered as an add-on to the cartoning process rather than an essential element in their own right.

The author reviews the state of downstream processing and considers potential solutions, including the streamlining of full processes and borrowed technologies.
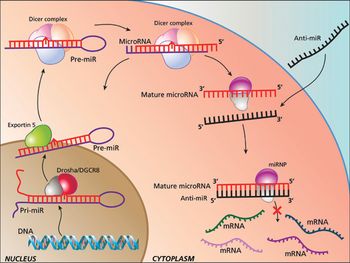
The authors provide further insight into microRNA biology, and the simplicity of anti-miR oligonucleotide drug delivery.
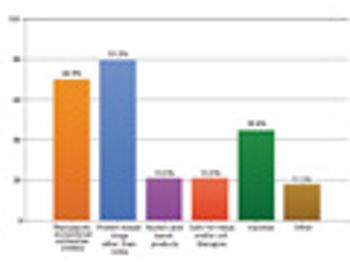
Results from our annual survey. This article contains online bonus material and is part of a special issue on Bioprocessing and Sterile Manufacturing.

The authors question certain aspects of the industry's current regulatory-compliance strategy and suggest that aseptic-process control and evaluation should be revised.

The author describes recent developments to help overcome the downstream processing bottleneck. This article is part of a special issue on Sterile Manufacturing and Bioprocessing.

Linking peptides to polyethylene glycol, or PEGylation, has helped improve pharmaceutical therapeutics in several ways. A wave of new techniques is now ushering in further advances.

A technical forum moderated by Patricia Van Arnum

Shanghai Pharmaceutical and Pfizer have signed a memorandum of understanding for the companies to jointly pursue potential business opportunities in China.

GlaxoSmithKline (GSK) identified certain over-the-counter (OTC) brands in its consumer healthcare business that the company plans to divest.