
The threat of counterfeit medicines in the next year will be more severe than ever, according to a survey of 1000 companies conducted in October 2010 by Pharma IQ.

The threat of counterfeit medicines in the next year will be more severe than ever, according to a survey of 1000 companies conducted in October 2010 by Pharma IQ.

Biopharmaceuticals commonly cannot be terminally sterilised, as such aseptic processing using sterilising grade filtration is essential.

UHPLC offers increased performance compared with HPLC, including shorter analysis times and increased sensitivity.

The authors describe a new method for the production of personalised medicines utilising ultra-precise microdrop printing technology.

GlaxoSmithKline (GSK) issued a statement on Jan. 2, 2011, to respond to a 60 Minutes segment that aired on Jan. 2 that focused on quality-control problems at a former GSK manufacturing facility in Cidra, Puerto Rico.

Gilead Sciences to Acquire Arresto Biosciences; Sartorius Appoints Head of Lab Business; and More.

US Senator Amy Klobuchar (D-MN) urged the US Food and Drug Administration and the pharmaceutical industry to address what she called an "unprecedented" shortage of prescription drugs, especially for chemotherapy.

FDA received the authority to mandate recalls of food under a new food-safety bill. Efforts are underway in the House to grant the agency this power for pharmaceuticals as well.

Pharmaceutical Technology discusses the key issues and trends facing the pharmaceutical supply chain with Bob Kanuga, executive director of the external manufacturing organization with Merck & Co. Inc. and president of the Drug, Chemical and Associated Technologies Association (DCAT).

The European Commission and Medicines Agency seem to be moving in advance of their ICH partners to update standards.
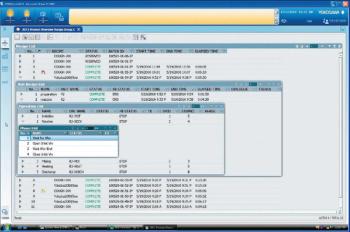
A look at manufacturing products in process control.

The authors discuss how their research will help FDA in its identification of areas of emphasis in pre- and postapproval evaluation of products and processes.

Industry executives share insight into the future direction of drug manufacturing and the supply chain. This article contains online bonus material.

The authors conducted an experiment to determine the type of extrusion that provides the best productivity and pellet quality. This article contains online bonus material.

Top priorities for manufacturers include user fees, new health initiatives, and regulatory compliance.
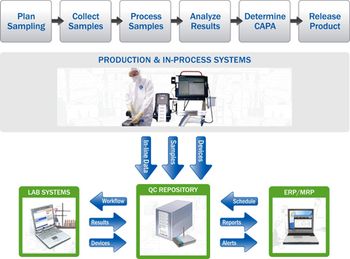
The authors discuss the role of quality-control automation in providing better data, enhanced compliance, and potentially faster release times.

The authors group key actions to consider when conducting a product recall and discuss how to execute them carefully and thoroughly.

Capable of great works, pharma as a whole still yields to the lesser angels of its nature.

Industry executives share insight into the future direction of drug manufacturing and the supply chain.

Developments in RNAi, monoclonal antibodies, and more are boosting the biotech marketplace.

A Q&A with Spectrum Laboratories President Tony MacDonald.

Holding product and supply-chain security to the highest standards is crucial for the future.
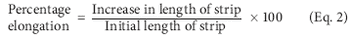
The manufacture of orally dissolving films is done by various methods such as solvent casting, hot-melt extrusion, semisolid casting, solid-dispersion extrusion, and rolling. The authors discuss these methods and the various parameters in which dissolving films are evaluated.

Poor organization makes it hard for readers to find the helpful information in a recent book.
Industry experts offer their best practices for dealing with deviations. This article contains online bonus material.