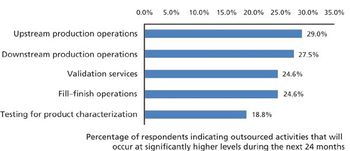
More crucial biomanufacturing operations are expected to be outsourced.

More crucial biomanufacturing operations are expected to be outsourced.

Anticounterfeiting solutions for vials and syringes.

Taking care to note, file and re-check information can save one from future mishaps.

How to adapt a real time release approach to powder processing during drug-product manufacturing.

The author describes why statistical significance would impose an unreasonable burden on manufacturers.
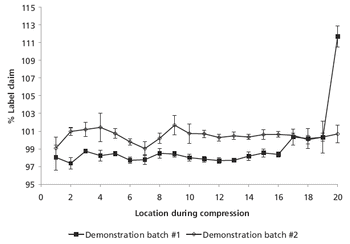
The authors modified equipment and the manufacturing process to re-establish content uniformity among tablets.

As the industry prepares for Informex, the trade show of custom and batch manufacturers in Charlotte, North Carolina, a roundup of key recent developments.

Innovations protect the quality of temperature-sensitive products.

A roundtable moderated by Angie Drakulich.

The authors highlight the need for a technology-transfer process that is efficient, cost-effective, and repeatable, stressing the importance of process understanding. Read this and other preferred organization articles in this special issue.

The article examines pharmaceutical market growth, company positioning, and the innovation potential in emerging markets. Read this and other preferred organization articles in this special issue.

A contract-service provider roundtable, featuring Albemarle, Baxter, DPT, Pfizer CentreSource, Dr. Reddy's, SAFC, and Vetter. Read this and other preferred organization articles in this special issue.

An interview with Vetter's Oskar Gold about the trends and growth in prefilled syringes.

The author analyzes the results of a survey that polled pharmaceutical executives and managers about both sides of the outsourcing relationship. Read this and other preferred organization articles in this special issue.

Pharmaceutical-industry executives from Merck & Co., Pfizer, and Covidien share their perspectives on their expectations and evaluation in the preferred-provider relationship. Read this and other preferred organization articles in this special issue.

Novartis Acquires Genoptix; Lilly Exec Becomes Savient CEO; and More.

Reflecting the manufacturing restructuring ongoing in the bio/pharmaceutical industry, Amgen and sanofi-aventis announced the sale of manufacturing facilities to contract manufacturers.

FDA published its long-awaited guidance titled Process Validation: General Principles and Practices this week.

Johnson & Johnson's run of quality issues, recalls and other bad news continues with yet another product recall.

Merck Teams with Parexel; Roche CFO to Retire; and More

Last Friday, French daily Le Figaro reported that sanofi-aventis intended to reach an acquisition agreement with Genzyme that would value the latter company at roughly $76 per share, or a total of $20 billion.

FDA Sets Limits for Acetaminophen in Prescription Combination Products

FDA issued a draft guidance for industry on Jan. 18, 2011, about the size of beads within drug products labeled for sprinkle.

GlaxoSmithKline is expecting to pay $3.4 billion to settle legal charges relating to its diabetes drug Avandia, as well as sales and promotional practices in the US for other products.

A company's contamination-control plan is an important document designed to formalize the rationale, methods, and validation of contamination-control procedures in a manufacturing facility. The author describes the role of bioburden in the contamination-control plan.