
Eli Lilly outlined its growth strategy at its annual meeting with the investment community last week.

Eli Lilly outlined its growth strategy at its annual meeting with the investment community last week.

Also, Abbott to acquire Starlims Technologies; GSK Biologicals to form alliance with Intercell.

PharmTech's monthly newsletter, Equipment & Processing Report, reviews the Editor's Picks for the December 2009 edition from Sepha and AdvantaPure.

Company and People Notes: Also, Pfizer and Protalix enter agreement; Watson acquires Arrow Group; and more.

The US Congressional Budget Office released a report that analyzes the pharmaceutical industry?s advertising strategies.

Personalized medicine has been described by analysts PricewaterhouseCoopers as a "disruptive influence" that will create both opportunities and challenges for traditional healthcare.
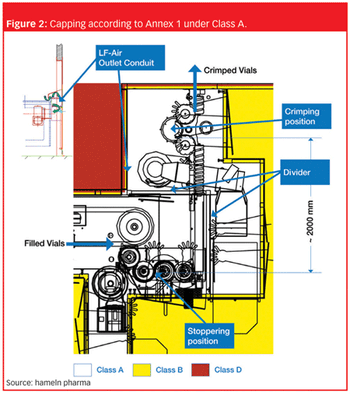
What makes a new sterile plant award winning?

The private industry has been urged to join a public sector commitment fund in the UK to advance manufacturing R&D in bioprocessing, following the investment of approximately £9 million from two UK research councils.

sanofi-aventis (Paris) announced last week that it has signed a memorandum of understanding (MoU)with Prominvest.

Novartis officially inaugurated its large-scale flu cell-culture vaccine and adjuvant manufacturing facility in Holly Springs, North Carolina.

The US Pharmacopeial Convention posted a proposed revised standard for what should and should not appear on the ferrules and cap overseals of medication vials.

Also, Merck & Co. extends collaboration with Idera Pharmaceuticals; Pfizer establishes R&D center in China; more...
In this article, the authors evaluated the effects of the granulating binder level, binder type, water amount, and water-droplet size on the MADG process.

Readers can learn about the importance of measuring and controlling water activity in a comprehensive new book.

Particularly in a more connected world, individual contributions can make a difference.

Editors' Picks of Pharmaceutical Science & Technology Innovations

Dumbed-down presentations and poor speaker selections are destroying a valuable industry tool.

With so many healthcare and pharmacy websites, consumers could use the agency's nod of support.

Gerry Migliaccio, senior vice-president of network performance for Pfizer Global Manufacturing, offers perspectives on building supply-chain integrity in emerging markets.

Blame it on cafeteria gossip, outdated procedures, and major miscommunication.

Generic-drug manufacturers look to expand into biologics and complex dosage forms. This article contains bonus online-exclusive material.
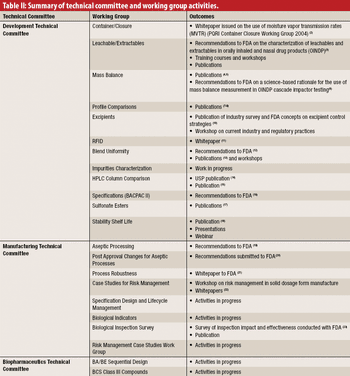
The authors describe the development and organization of the Product Quality Research Institute and highlight some of the important projects conducted by its Working Groups.

When it comes to quality assurance and quality control in pharmaceutical solid dose manufacture, Fourier-Transform Near Infrared spectroscopy may be the answer.

The quality assurance environment is forcing pharmaceutical companies to face new challenges. In light of this, the authors conducted a study of professionals from some of the world's top pharmaceutical companies to identify key QA concerns.

Manual sample preparation methods for solid dose pharmaceuticals have a number of inherent disadvantages, primarily because they are time consuming and unpredictable.