
If both sides of the aisle don't agree on even mild healthcare reform soon, the bill could die out.

If both sides of the aisle don't agree on even mild healthcare reform soon, the bill could die out.

The vice-president addresses shifts in process and more.
Technological developments make it easier to manufacture sterile parenterals.

Visitors to INTERPHEX 2010 will find an abundance of packaging innovations. Plus: Check out our preliminary program for LIVE video interviews at the show.

The author reviews the benefits, challenges, and considerations to be made when selecting a gamma-irradiation sterilization method.

Although efforts have been made to improve solubility, many poorly soluble drugs still reach the market. Peter Nielsen discusses new developments in solubilisation technology, which could help those companies seeking to reinvigorate marketed products.
The authors describe an industrialized process for the manufacture of iPSC-derived human cardiomyocytes. This article is part of a special issue on Bioprocessing and Sterile Manufacturing.

Single-use technologies can help simplify equipment and facility design, thus bringing cost advantages. But how are these cost advantages realized and what criteria can be used to assess their impact? This article is part of a special issue on Bioprocessing and Sterile Manufacturing.
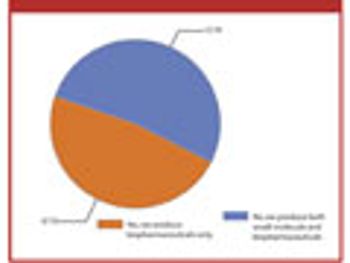
The second annual Pharmaceutical Technology Bioprocessing Survey offers a snapshot of the industry following 2009's megamergers.

The author examines the use and advantages of disposable technologies in the fill–finish of sterile pharmaceutical products and how these technologies can reduce costs and time in producing clinical-trial materials.

Pharmaceutical Technology talked to drugmakers, equipment vendors, and service providers to learn more about the advantages and disadvantages of rapid microbial-detection methods.

The authors propose a process-centric approach for carrying out aseptic-processing and suggest further dialogue. This articles contains bonus online-exclusive material.

Freeze drying is widely used in pharmaceuticals to improve the long-term storage stability of labile drugs.

A Q&A with GE Healthcare

The authors propose increased use of single-use technologies in biopharmaceutical manufacturing to achieve operational excellence without compromising product quality.

Moisture-activated drying granulation is a simple and effective process for particle size enlargement.

The most important consideration when choosing a freeze dryer is to ensure the system is fit for both today's applications and future needs.

Pharmaceutical Technology Europe discusses some of the latest challenges and innovations in pharmaceutical lyophilization.

Last year, a member of the PTE editorial team attended the 2nd Annual Lyophilisation Conference in London (UK).

Could a collaborative approach involving both public and private parties offer a solution to Europe's lack of innovation?

Pharmaceutical Technology Europe spoke with experts about the developments that have shaped the nanotechnology industry today and the benefits that this technology has to offer the pharmaceutical industry, which remain largely untapped.

A significant increase in the recruitment of contract staff is expected during the next 12 months in the UK biotechnology sector, according to a survey conducted by SRG, which provides personnel to various science-based sectors.

A significant increase in the recruitment of contract staff is expected during the next 12 months in the UK biotechnology sector, according to a survey conducted by SRG.

Eli Lilly, Merck Sharp & Dohme, and Pfizer form the Asian Cancer Research Group, a nonprofit aimed at accelerating research and improving treatment for patients affected with the most commonly diagnosed cancers in Asia.

Merck To Eliminate 15,000 jobs; Tauzin Stepping Down At PhRMA; And More.