
A panel of industry and regulatory experts, including FDA, discuss efforts to secure the global pharmaceutical supply chain. This article contains bonus online-exclusive material.

A panel of industry and regulatory experts, including FDA, discuss efforts to secure the global pharmaceutical supply chain. This article contains bonus online-exclusive material.

Contract organizations waiting for the pipeline to come roaring back are kidding themselves.

New tests solve one issue, but cheaper plastic and new stoppers cause problems.
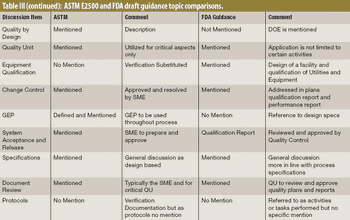
The author explores differences between two qualification documents, the draft guidance from FDA "Process Validation: General Principles and Practice" and the ASTM E2500-7 standard "Guide for Specification, Design, and Verification of Pharmaceutical and Biopharmaceutical Manufacturing Systems and Equipment."

The heightened focus on risk raises concerns about delays in approving new drugs.

While Congress debates hundreds of healthcare plan proposals, perhaps we, the public, can get in the game too.

Functionalized supramolecular catalysts and an enantioselective route to unnatural amino acids are some recent developments.

A recent book reminds readers that small-molecule chemistry has enabled advances in biotechnology.

The authors developed a robust, automated system to conduct Karl Fischer moisture assays for lyophilized products.
The authors explain a process for moisture-activated dry granulation in detail and provide guidance for the selection of excipients and equipment.

FDA's Edwin Rivera-Martinez on dodging supply-chain challenges.

Editors' Picks of Pharmaceutical Science & Technology Innovations

New pricing controls and healthcare reforms may be pushing the pharmaceutical market out of this southeast Asian country. This article contains bonus online-exclusive material.
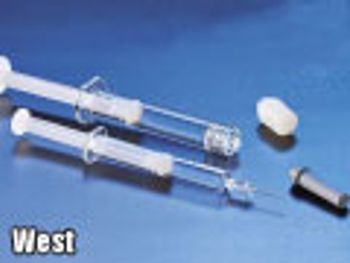
The authors describe the ways in which plastic prefilled syringes can be an alternative that provides consistent performance, protects drugs prone to degradation, and enhances patient safety.

The author describes how Merrion Pharmaceuticals reformulates parenteral drugs into tablets and capsules that are easier for patients to take and provide better bioavailability.

The author details the factors in formulation design, requirements in facilites and equipment, and validation criteria for aseptic formualtions.

A Conversation with Greystone Associates' George Perros. This article contains bonus online-exclusive material.

The author discusses the risks involved with aseptic processing, methods and tools used to identify and control risk, and regulatory guidelines relevant to the risk-management process.

A Conversation with Otonomy

According to a survey conducted by Pharmaceutical Technology Europe (PTE), almost 50% of you believe that cost is the biggest limiting factor to innovation in the pharmaceutical packaging industry.

Following years of development, DataMatrix codes are now a viable labelling solution for pharma manufacturers.

Michael Rubenstein, Chief Growth and Innovation Officer at Alcan Packaging, talks about applying a philosophy of sustainability to the pharmaceutical packaging supply chain.

Population ageing is an issue often discussed in the media, but its impact on all facets of life has been poorly characterized.

In 2010, Braille embossing will be required on all European pharmaceutical packages. Is the industry ready?

Apopular definition of insanity is repeating the same task over and over again while expecting a different result. It would appear, therefore, that many people in the contract services industry think the major global bio/pharma companies are insane.