
The working acceptance limits for acceptance values (AV) are determined using the critical values at, for example, 95% coverage over the corresponding AV distributions. However, validity of such limits needs to be elaborated.

The working acceptance limits for acceptance values (AV) are determined using the critical values at, for example, 95% coverage over the corresponding AV distributions. However, validity of such limits needs to be elaborated.

Shipping biopharmaceuticals in single-use containers requires a thorough understanding of the distribution cycle and potential transportation risks.

Increased use of single-use systems has led to a need to redefine safe, stable and integral systems for shipping biopharmaceuticals around the world. This article provides qualification data under international ASTM D4169 norms.

As Astellas Pharma winds down its Agensys research operations, the company sells the Santa Monica, CA, facility to Gilead.

The event, taking place on June 6–7, 2018 in Clinton and Somerville, NJ, will focus on the latest melt extrusion and granulation technologies for pharmaceutical and nutraceutical products.

The company will showcase its drum-filling technology among other products and services at the upcoming ACHEMA 2018 event in Frankfurt, Germany.

Proteus Digital Health is collaborating on a pipeline of digital, oral solid-dosage drugs for various therapies, including cardiovascular and oncology drugs, based on its first NDA, which used ingestible sensors in an antipsychotic treatment.
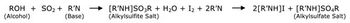
Understanding the advantages and suitability of different methods to measure residual moisture content in lyophilized materials-and the respective limitations-aids in selecting the most appropriate method for testing.

Thermo Fisher Scientific invests $50 Million to expand biologics drug development and manufacturing capabilities in St. Louis, MO.

The new platform is expected to speed up cell line development.

The company unveiled three new products to support single-use biomanufacturing and won an award for best technological innovation at INTERPHEX 2018.

INTERPHEX presents advances in pharmaceutical container filling, robotic handling, and serialization.

The company is launching a new pre-fabricated, ready-to-run manufacturing facility that is expected to significantly decrease the production timeline for viral vector-based therapeutics.

Honeywell’s Experion Batch visualization technology provides operators with improved visibility, simplified maintenance, and faster validation.

Sartorius Stedim Biotech will supply equipment for Penn State’s new Fermentation Facility in the university’s Center of Excellence in Industrial Biotechnology.

Under an Innovate UK grant for a three-year project, Arcinova and the University of Nottingham will develop a continuous, flexible modular manufacturing technology platform.

A new report gives an overview of the work of the International API Inspection Program.

This workshop, to be held in Sheffield, United Kingdom, on June 7, 2018, will provide an introduction to the use of glass as primary pharmaceutical packaging.

A $5.5-million expansion at its Philadelphia, PA clinical supplies facility gives Catalent additional packaging and storage capacity.

The company will build a high-volume manufacturing facility for their glass packaging product in Durham County, NC.

The Parenteral Drug Association’s Data Integrity Task Force helps industry members understand regulatory requirements for data integrity.

The contract development and manufacturing organization will discuss its capabilities in topical drug products at CPhI North America, taking place April 24–26, 2018 in Philadelphia, PA.

The new facility, to be built in Toronto, Canada, will significantly increase capacity for pediatric and booster vaccines.

New filling and inspection machines and new containers and closures for injectable drugs will be on display at INTERPHEX 2018.

The biopharmaceutical firm has chosen Rhode Island as the site of its next-generation biomanufacturing plant, which will offer flexibility, speed, and efficiency.