
The company plans to install 4000-L disposable bioreactors from ABEC at its new commercial manufacturing facility in Wuxi city, China.

The company plans to install 4000-L disposable bioreactors from ABEC at its new commercial manufacturing facility in Wuxi city, China.

Helsinn will acquire the global rights to Valchlor/Ledaga (mechlorethamine/chlormethine), a topical skin-cancer drug, from Actelion Pharmaceuticals.

Process analytical technology and advanced process control strategies can be applied to either batch or continuous manufacturing of solid-dosage drugs.

ISPE announced BioMarin Pharmaceutical, Shire, Vetter, and Wyeth Pharmaceuticals as FOYA Category winners.

An expanded pharma services supply chain facility in Germany boosts Thermo Fisher’s Pharma Services footprint in Europe.

Puncture and aerosolization tests measure the effectiveness of hard-shell capsules used in dry powder inhalers (DPIs) for inhaled drug products.

Hovione plans to continue to expand its sites in Portugal and Ireland for chemical synthesis, spray drying, and laboratory analysis.

Biogen will acquire an AMPA receptor potentiator for cognitive impairment associated with schizophrenia in a deal worth approximately $590 million.

Sartorius Stedim Biotech has launched a new automated parallel bioreactor system for perfusion culture.

Sartorius has delivered to ABL’s Strasbourg facility, a GMP viral vector manufacturing package solution that includes single-use bioreactors and an automation platform for normal flow filtration, tangential filtration, and mixing.

The CDMO’s new blow-fill-seal machinery at its Kaysersberg site is now operational.

The contract development and manufacturing organization has expanded biologics capacity at its facility in Berkeley, CA.

The delivery device and drug form should be considered when choosing a test method for identifying and measuring particulates.

A previously published article presented difficulties with the revised European guidelines on sterile manufacturing. The authors included a brief summary of the comments developed on the draft document. This article expands upon that summary, outlines the authors' rationale, and highlights the most difficult aspects of the revision draft.

Revisions to chapters on glass containers and elastomeric closures were canceled following review of comments.

The Natoli AIM Data Acquisition and Analytical Software for Natoli’s NP-RD10A single-station tablet press speeds research and development.

Machine vision and optical character recognition technologies enhance inspection of packages and labels.

GlobalData reports the need to shift away from egg-based manufacturing of vaccines in light of influenza-related deaths.

Under this agreement, the companies will develop in parallel an antibody drug candidate and cell lines for other potential candidates.

With minimal damage to its manufacturing plant in Humacao, Puerto Rico, Colorcon has been able to restore full operations.

Grand River Aseptic Manufacturing announces first planned investment in capacity expansion.

In selling its contrast media business, Hovione will focus on API and drug product development and manufacturing.

This article presents considerations for preventing hazards associated with dust in pharmaceutical production, particularly as relates to the United Kingdom's Control of Substances Hazardous to Health (COSHH) Regulations.
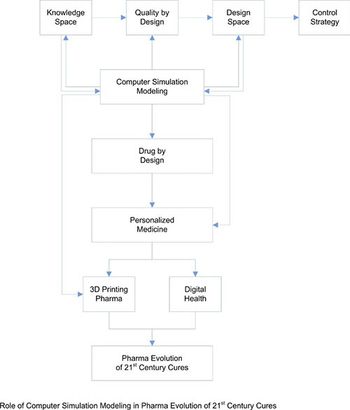
Twenty-first century drug development challenges sponsors and contract partners to collaborate more closely, using tools such as model-based simulation.

JLL, a real estate and investment management firm, recently released a new report on three trends shaping labs for the future of life sciences R&D.