Quality Systems
Latest News
Latest Videos

More News

The new lab will be used to develop and scale innovations for the production of medicines and to help secure the supply chain.

EMA has published recommendations to address potential radiopharmaceutical shortages.

Matt Cushing, VP of Quality and Science, Nelson Labs, and Susan J. Schniepp, distinguished fellow at Regulatory Compliance Associates, a Nelson Labs Company, discuss PDA/ANSI Standard 06-2025: Assessment of Quality Culture Guidance Documents, Models, and Tools, which was published in February 2025.

A Phase III trial demonstrated mNEXSPIKE’s non-inferior efficacy compared with Moderna’s original COVID vaccine, Spikevax.

Pharmaceutical Technology® spoke with Martin Meeson, CEO of Axplora, about the role contract development and manufacturing organizations have in ensuring the quality of APIs and the security of the supply chain.

Teva’s investigational monoclonal antibody, TEV-53408, has been designed for use in adults with celiac disease, for which a strict and lifelong gluten-free diet is currently the only treatment.

Both companies viewed the early end to the PIVOT-PO trial as a positive development, with GSK saying it would work with US regulatory authorities to move the treatment forward in 2025.

Leveraging computerized maintenance management systems software can enhance efficiency, improve quality control, ensure accurate documentation, and strengthen data integrity.

In this episode of the Ask the Expert video series, Susan J. Schniepp, distinguished fellow at Regulatory Compliance Associates, and Siegfried Schmitt, PhD, vice president, Technical at Parexel, discuss how to address an FDA warning letter citation for a failure to establish a quality control unit.

Poor API quality may often lead to delays in production and a shortage of supply.

Calquence (acalabrutinib) plus bendamustine and rituximab reduced risk for disease progression or death by 27% compared with the current standard of care, according to a phase III trial.

FDA chooses Dr. Vinay Prasad, MD, MPH, a hematologist-oncologist, to lead the Center for Biologics Evaluation and Research at FDA.
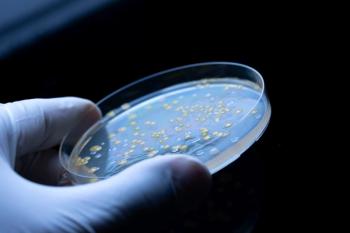
This article explores the impact of test volume, microbial distribution, and dilution errors on bioburden testing variability. It presents statistical approaches to estimate percentage error and discusses strategies to optimize microbial enumeration techniques in biopharmaceutical quality control.

Amit Chivate, senior market manager, Greater Asia and China, Roquette, provides his perspective on FDA’s recommendations regarding nitrosamines.

The White House is instructing FDA to increase fees for and inspections of foreign drug manufacturing plants and reduce the time required to approve such sites that will be newly constructed in the US.

The company said the expansion is in response to globally rising demand for inhaled biologics, which offer distinct advantages in route of administration, but can be challenging products for developers.

The European Union is discussing ways to reduce Europe’s over-reliance on imports of APIs.

While global harmonization exists, there are still differences between the US and European GMP requirements that manufacturers should know, says Siegfried Schmitt, PhD, vice president, Technical at Parexel.

Aviva Capital Partners and developer Socius are investing £1 billion to develop a cancer research and treatment center in Sutton, London.

The pharma industry is focused on strengthening its foundation, embracing innovation, and future-proofing its path forward.

Bempikibart (ADX-914) is a human anti-IL-7Rα antibody that blocks the IL-7 and TSLP pathways, which have been implicated in driving T cell-mediated pathological processes in autoimmune diseases.

Pharmaceutical Technology® spoke with Alexander Seyf, CEO and co-founder of Autolomous, about the impact geopolitical changes in Europe might have on the bio/pharmaceutical industry.

Pharmaceutical Technology® spoke with Alexander Seyf, CEO and co-founder of Autolomous, about how the political landscape may impact the pharmaceutical industry.

The company said that Vyvgart (efgartigimod alfa) is the first novel mechanism of action for CIDP treatment in more than 30 years.