
The agency put Baoying County Fukang Medical Appliance Co., Ltd. on import alert after observing violations at the company’s Yangzhou City facility.

The agency put Baoying County Fukang Medical Appliance Co., Ltd. on import alert after observing violations at the company’s Yangzhou City facility.

A pilot project, beginning in 2017, will support the development of biosimilars.

The companies entered a manufacturing agreement for the future commercial production of Lenti-D and LentiGlobin product candidates.

Emergent signed a follow-up contract to provide 29.4 million doses of BioThrax to the Strategic National Stockpile.

The agency announces that 81 medicines overall were recommended in 2016.

The commission concluded its P4Bio pilot phase with the adoption of the monograph for etanercept.

FDA’s Center for Drug Evaluation and Research makes plans for implementation of the 21st Century Cures Act that include patient-focused drug development.

This article looks at five of the most common hurdles in packaging and labelling artwork and how to avoid them.

Congress enacted the 21st Century Cures legislation, which shores FDA operations and supports biomedical research at the National Institutes of Health.

The integration of a method validation, transfer, and verification process into the overall lifecycle management process of a product can best align the variability of the analytical procedure with the requirements of the product.

FDA issued a warning letter to Interquim, SA for CGMP deviations at its Barcelona API facility.

Dara Corrigan examines the Mutual Reliance Initiative as a method for expanding FDA’s inspection capabilities in Europe and beyond.

Susan Schniepp, distinguished fellow at Regulatory Compliance Associates, discusses training personnel on a limited budget.

Airlocks, gowning rooms, and transition spaces have different uses and should be considered separately in cGMP pharmaceutical facility design.

Pharmaceutical companies must see regulators as partners in their efforts to provide safe and effective therapies worldwide.

Pharmaceutical Technology reached out to the US Pharmacopeial Convention (USP) to get an understanding about how pharma manufacturers can get involved in developing industry standards.

Susanne Keitel, director of the European Directorate for the Quality of Medicines and Healthcare (EDQM), discusses the role industry plays in the development of pharmaceutical standards.

Better co-ordination within and between regions is needed to improve the global regulation of medicines, according to the European Medicines Agency.
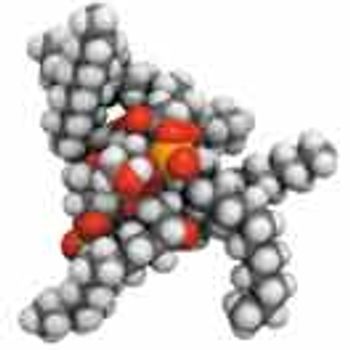
Understanding of endotoxin assays and a range of detection technologies are essential for effective testing.

Republican control of Washington promises overhaul of healthcare and medical product regulation.


The commission approved future plans, appointed members, and adopted texts during its November 2016 session.

FDA has revised its draft guidance to provide greater clarity and focus. This version would establish an initial voluntary phase to allow regulators and industry to focus, first, on a limited set of metrics.

Wockhardt Limited received a warning letter from FDA for CGMP violations.

FDA issued a warning letter to Srikem Laboratories Pvt. Ltd. for data integrity violations