
GenPak Solutions is cited by FDA for cGMP violations at its Hilliard, Ohio facility.

GenPak Solutions is cited by FDA for cGMP violations at its Hilliard, Ohio facility.

Generic versions of AstraZeneca’s blockbuster Crestor will hit the US market after a federal judge refused to issue a restraining order blocking the approval of rosuvastatin ANDAs.

The committee voted unanimously in favor of approving the drug, but the majority supported implementing additional risk management.

The company is voluntarily recalling a docusate sodium solution distributed by Rugby Laboratories due to risk of contamination.

FDA issued a warning letter to the Worthing, UK facility for cross contamination and microbial contamination cGMP violations.

The agency says the increasing requests for orphan drug designation has resulted in a change in FDA’s review goals.

The agency reviews hemophilia A, skin, and diabetes treatments, among others.

The agency completes its risk assessment of the blood cancer treatments.

The agency announced that it has completed the review of the GDUFA backlog one year ahead of schedule.

The agency says that the routine large-scale compounding of drugs that are exact copies of existing medications undermines the the drug approval process.

The draft guidance addresses control of elemental impurities in harmonization with implementation of ICH Q3D guideline.

FDA cited Guangzhou Haishi Biological Technology Co., Ltd. with CGMP violations.

Agency guidance and industry standards aim to reduce lapses and improve quality operations.
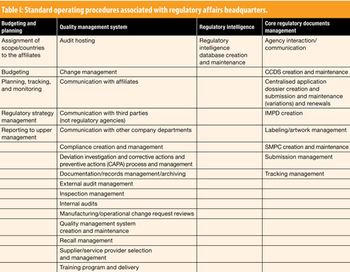
Siegfried Schmitt, Principal Consultant, PAREXEL International, discusses standard operating procedures for the regulatory affairs department.

Designing systems using the principles of good documentation practice, including validated audit trails, is a key piece of a manufacturing data integrity program.

Early planning for the integration of clean-in-place systems for equipment cleaning is key.

FDA approved the Raplixa, the first spray-dried fibrin sealant, in May 2015 to help control bleeding in adults during surgery.

China and India are also increasing inspections and becoming more exigent about data integrity and cGMPs.

Data integrity and cGMP issues demand closer scrutiny of suppliers. Bribery and corruption may become the next supply chain flashpoint.

The impact of pharmaceutical manufacturing on the environment has triggered demands for tighter environmental controls in EU and national legislations.

The drug received breakthrough therapy and orphan drug designation as a monotherapy for the treatment of chronic graft-versus-host-disease.

The monoclonal antibody for the treatment of two forms of multiple sclerosis has a target action date of December 28, 2016.

ICH detailed the highlights of the council’s June 2016 meeting.

The agency is following up on a February 2016 inspection of the facility that found GMP violations.

The agency recommends Zalmoxis, a new cell-based therapy to support stem cell transplantation in patients with high-risk blood cancer.