
The agency cited the company for sterile manufacturing violations.

The agency cited the company for sterile manufacturing violations.

The two agencies have set up a working group on involving patients in drug development.

The assay, co-developed by Grifols and Hologic, will be used to test blood donations in the United States.

Emergent is seeking approval for the manufacture of BioThrax at the company’s large-scale manufacturing facility.


The OMCL Network met increasing market demand for testing quality of medicinal products, blood-derived medicinal products, and vaccines in Europe.

The agency provides quality, development, manufacturing, and labeling recommendations.
Computerized systems can solve some of the data integrity problems with conventional paper-based systems.

The agency cited a Memphis drug compounder for misbranded drugs and a lack of appropriate sterile processes.

The issues cites the Taiwan facility with violations of current good manufacturing practice regulations.

FDA cited a Las Vegas compounding pharmacy for sterility violations.

FDA sent a warning letter to The Compounding Pharmacy of America for deficiencies in sterile manufacturing.

The agency published guidance on the nonclinical evaluation of osteoporosis treatments.

FDA approved Vaxchora intended for travelers who are at risk for the disease.

Mandatory use of the periodic safety update report repository becomes mandatory on June 13.

Sharing un-redacted inspection reports between FDA and EMA may reduce duplicate inspections of facilities.

The agency publishes three final guidance documents on drug compounding.

The agency cited KO DA Pharmaceutical Co. with cGMP violations.

There are technical hurdles to clear in developing serialization and track-and- trace programs, but it’s the human ones that are proving most difficult to surmount.

The agency reiterated its earlier decision to require suffixes in biosimilar naming, but was unclear on suffix meaning.

The agency published a report on fostering the development of advanced therapy medicinal products.
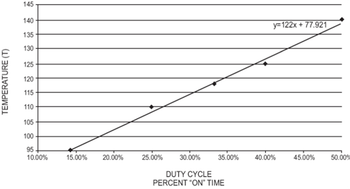
Preheating pinch valves prevents drift in the volume of liquid dispensed.

FDA and bio/pharma companies get serious about continuous manufacturing to ensure product quality.

Industry experts discuss common considerations and recent technological advancements in blow-fill-seal technology.

Investment in talent and infrastructure, more quality-by-design skills, and increased communication with global regulators will be needed to combat compliance issues at API and drug manufacturing facilities in India.