
The agency has announced the creation of the Combination Products Policy Council to address issues related to combination products.

The agency has announced the creation of the Combination Products Policy Council to address issues related to combination products.

The agency released a draft reflection paper on the extrapolation of data from adults to children in the development of pediatric drugs.

Jack Lew, Obama’s secretary of the treasury, announced on April 4, 2016, that the US Department of the Treasury and the Internal Revenue Service (IRS) is issuing temporary and proposed regulations to limit the “benefits of and limit the number of corporate tax inversions.” The government bodies also plan to address earnings stripping in these inversions, so it will examine past inversion deals that have already been completed.

Texture analyzers can be used to evaluate wall hardness and elasticity of softgel capsules.

Non-destructive container closure integrity testing allows 100% inspection of all ampoules, syringes, vials, and cartridges.

Consider the 3 Ps (product, process, and patient) when choosing a parenteral packaging material.

Susan Schniepp, distinguished fellow, and Andrew Harrison, chief regulatory affairs officer and general counsel, both of Regulatory Compliance Associates, discuss requirements for successful product technology transfer.

Industry experts and FDA’s Office of Pharmaceutical Quality discuss the challenges, trends, and regulations involved in ensuring quality in solid and semi-solid dosage forms.

Understanding of the risks associated with FMEA is crucial in lot release testing.

A global API marketplace increases the burden of supply chain monitoring for drug companies.

The authors discuss the concept design of a versatile, sustainable, small-scale facility in Malta that conforms to the European Union’s current good manufacturing practices.
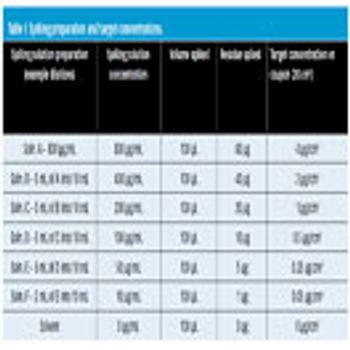
Visible residue limits have been shown to be a valuable tool in validated cleaning validation program.

Global outbreaks energize vaccine R&D and drive production modernization.

Pharmaceutical Technology spoke with FDA to get the agency’s insights on how the industry can ensure quality in solid and semi-solid dosage products.

The European Commission publishes regulations on mandatory packaging safety features to fight counterfeiting.

The European Medicines Agency report provides an analysis of the health-technology assessment pilot program, which began in 2010 and completed in March 2016.

The draft guidance states information concerning a clinical study of a biosimilar should only be included if it is necessary to demonstrate the safety and efficacy of the drug.

Pharma has huge responsibilities resting on its shoulders to deliver safe and effective medicine.

Cinqair is approved for patients that have a history of severe asthma attacks despite receiving their current asthma medication.

The drug is marketed by Eli Lilly in the US, and will be available at the beginning of the second quarter of 2016.

The report examines the increased number of companies cited by regulators for data integrity issues.

The draft guidance outlines ways applicants can test for abuse deterrence in solid oral opioid drugs.

The BARDA-supported monoclonal antibody was approved both as a treatment after anthrax exposure and as an anthrax prophylactic.

The agency announced enhanced warnings for immediate-release opioids to inform prescribers and patients of risks related to use.

Hospira recalls one lot of 8.4% Sodium Bicarbonate Injection, USP, due to particulate matter found within a single-dose glass fliptop vial.