
The author discusses how x-ray spectrometry can be an effective way to measure these impurities and meet regulatory guidelines.

The author discusses how x-ray spectrometry can be an effective way to measure these impurities and meet regulatory guidelines.

The authors present analysis of the state of control of intermediate identity and quality, based on analysis of recently submitted DMFs.

Both agencies warn of increased rates of serious adverse events, including death, during clinical trials of Zydelig (idelalisib).

Wear-resistant materials and coatings protect tablet punches and dies from abrasive formulations.

Doctors and oncologists agree with pharma companies in opposing the latest proposal from the CMS to revise how it pays for drugs administered in doctors’ offices.

The company recalls one lot of amikacin sulfate injection USP, 1 gram/4mL (250 mg/mL) vials due to the potential presence of glass particulate in one vial.

The group argues that the so-called “incentives” for physicians to prescribe more costly medications are essentially non-existent following a handful of prior cuts to Medicare Part B reimbursement.

The five-year Medicare initiative seeks to change the way in which drugs are reimbursed under Medicare Part B.

Sagent Pharmaceuticals issues a nationwide recall of Fluconazole injection, USP, due to out of specification impurity results.

The agency strengthens its support for treatments of unmet medical needs with launch of new PRIME scheme.
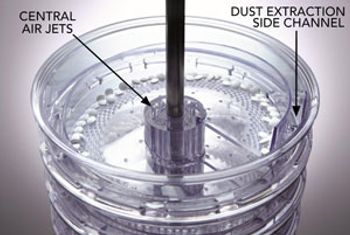
Dedusting equipment and techniques address problems associated with tablet manufacturing dust accumulation.

The inspection confirmed that the facility was compliant with GMP guidelines.

Policy makers debate strategies for promoting access to less costly medicines.

Siegfried Schmitt, principal consultant, PAREXEL, discusses how the regulatory requirements for CGMPs is the different phases of drug development and manufacture.

Data integrity is a widespread, global problem that must be addressed.

Standardized testing protocols are crucial for acceptance of single-use systems.

The EU is striving to reduce the costs and administrative burden facing pharmaceutical manufacturers when complying with variations regulations for keeping authorization dossiers up to date.

Data integrity has become a more serious compliance problem at pharmaceutical manufacturing plants throughout the world.

The program is intended to provide support to ongoing efforts in rare disease product development.

The agency has revised its good pharmacovigilance practices guide on risk management systems.

Tablet presses require regular inspection and maintenance to prevent premature wear and tableting problems.

Manufacturing of antibody drug conjugates requires high-containment solutions, such as high-performance aseptic isolators.

Regular removal of residues from disinfectants and sporicidals is important for improved aesthetics and safety in cleanrooms.

The directorate is looking for experts to join the European Pharmacopoeia network.

The agency published guidance on immunogenicity-related considerations for low molecular weight heparin.