Quality by design, in-vitro release testing, and modern analytical methods are improving understanding and control of these complex formulations.

Quality by design, in-vitro release testing, and modern analytical methods are improving understanding and control of these complex formulations.

The results of an industry workgroup’s examination of EMA’s guide on shared facilities are presented.

While some industry trade groups are gunning for distinct, nonproprietary names for biosimilars (mostly providers, brand manufacturers, and patient advocacy groups), and others for common names (mostly biosimilar manufacturers, insurers, pharmacies, and the Federal Trade Commission [FTC]), some are requesting that no decisions be made at all at this stage in the game. Express Scripts, a large pharmacy benefit manager (PBM), submitted a comment to FDA asking that the regulatory agency hold off on naming decisions until it releases formal guidance on interchangeability first.

The agency promotes safer use of drugs and prevention of medication errors through a new webpage and practice guide.

The Safe Chain Track and Trace System automates traceability for supply chain security.

Cambridge Consultants engaged in a workshop-style dialogue with a cross section of senior personnel from both Indian and multinational pharma companies to debate whether emerging markets are an opportunity to drive sustainable growth. Conclusions from the workshop are presented in this article.

Pharmaceutical manufacturing facilities can help prevent contamination and cross contamination by using color coding.

FDA emphasizes the surveillance aspects of quality metrics to concerned drug manufacturers.
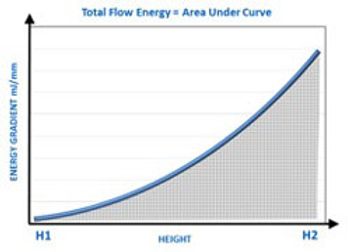
Dynamic powder testing and measurement of bulk powder properties can complement shear cell testing to identify the causes of poor hopper performance in solid-dosage drug manufacturing.

Robert Califf addresses questions about drug pricing at the Senate hearing to weigh his appointment to be the next commissioner of FDA.

Operations at Catalent’s Beinheim, France, softgel facility were suspended following suspected deliberate action to misplace capsules.

The agency issues guidance on the labeling of over-the-counter products that contain acetaminophen.

The new executive director of the European Medicines Agency begins appointment.

FDA seeks feedback on possible analytical standards and approaches to optimize regulation of next-generation sequencing (NGS)-based in vitro diagnostic tests.

Even though rising production and use of generic pharmaceuticals is saving billions for the nation’s healthcare system, policy makers continue to slap the industry with policies it claims will limit product development and sales.

The European agency presents guidelines for conducting post-authorization efficacy studies.

FDA noted multiple manufacturing, testing, and labeling violations at American Family Pharmacy’s Indianapolis facility.

New program emphasizes quality, risk, and global collaboration.

Siegfried Schmitt, principal consultant, PAREXEL, discusses how to ensure archive records can be retrieved.

Virtual pilot programs examine scenarios that may occur while implementing serialization requirements for the US Drug Supply Chain Security Act.

The scheme aims to ensure that EMA and licensing authorities of EU member states will use the same IT system, based on a single data standard.
The global supply chain for bovine and porcine heparin and regulatory considerations are examined.

Although shortages, quality, and regulatory challenges remain, improved technologies and new investments suggest that the worst may be over.

Miriam Beyer, European marketing manager, West Pharmaceutical Services, describes causes of recent parenteral drug shortages.

The company is voluntarily recalling the epinephrine injection, USP because of potential inaccurate dosage delivery.