
FDA issued a warning letter to Zhejiang Hisun Pharmaceutical Co., Ltd., as a result of inspections that took place on March 2–7, 2015 at the Taizhou City, Zhejiang Province, API manufacturing facility.

FDA issued a warning letter to Zhejiang Hisun Pharmaceutical Co., Ltd., as a result of inspections that took place on March 2–7, 2015 at the Taizhou City, Zhejiang Province, API manufacturing facility.

A US district judge entered a consent decree of permanent injunction on behalf of FDA, between the United States and pharmaceutical company, Downing Labs.

FDA approved 45 novel new drugs in 2015, the highest number of approvals since 1996 and second-highest ever.

The Office of Prescription Drug Promotion issues all-time low number of violation letters in 2015.

FDA issues a Warning Letter to Cadila Healthcare Limited for cGMP violations.

The company has voluntarily recalled one lot of magnesium sulfate in water for injection because of incorrect labeling.

FDA discusses a new program that allows pharmaceutical companies to submit proposals for new manufacturing technology.

Baxter voluntarily recalls two lots of intravenous solutions due to particulates.

More “me-betters” and more focused breakthroughs could enhance new drug development.

The bio/pharmaceutical industry will face increased scrutiny of product quality and cost drivers.
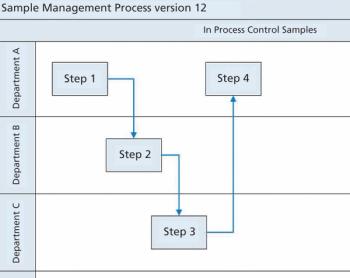
Siegfried Schmitt, principal consultant, PAREXEL, discusses how to streamline the document management process during market expansion.
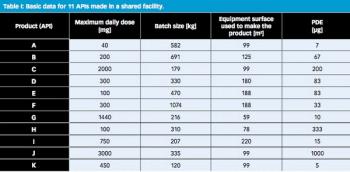
The 10-ppm criterion for the acceptable concentration of potential API in cleaning validation to minimize cross contamination into next product has been employed for many years. This article describes why the 10-ppm criterion, which was established based on analytical limitations and estimates of acceptability, is no longer necessary and why a risk-based approach should be universally adopted.

Amid corporate restructurings, regulatory initiatives, and aging R&D assets, will drug development accelerate or stall in 2016?

Leading nations are backing moves to strengthen WHO’s central role in international health security following the Ebola crisis, which sparked criticisms on the organization’s ability to address a pandemic outbreak.

Inadequate response to violations cited in a 2014 inspection leads to an FDA warning letter for Sun Pharmaceutical Industries.

The FDA program that encourages biopharma companies to develop new treatments for rare and neglected diseases has been in the spotlight recently.

The agency has published draft guidance on safety assessment for investigational new drug application safety reporting.

Janet Woodcock, director of the Center for Drug Evaluation, highlights FDA's priority list for 2016.

The agency has launched a new web platform to foster scientific innovation.

The use of drug compounding facilities to produce over-priced generic drugs raises quality and regulatory questions.

FDA warns the industry of possible contamination in the API baclofen from Taizhou Xinyou Pharmaceutical & Chemical Co., Limited.

EMA Executive Director Guido Rasi outlines his plan for the agency, including a focus on R&D.

Changes in China’s Food and Drug Administration (cFDA) drug development and commercialization policies make it easier for multinationals and CMOs to manufacture in China for in-country use, reports CMO and consultant PaizaBio.

The United States Pharmacopeial Convention announced the recipients of the 2015–2016 Global Fellowship Awards.

While stakeholders generally welcome improvements to quality initiatives, they are concerned with how the new requirements will be implemented for more complicated supply-chain models.