
The agency has released guidance on bioequivalence studies for asenapine, prasugrel, sitagliptin, and zonisamide.

The agency has released guidance on bioequivalence studies for asenapine, prasugrel, sitagliptin, and zonisamide.

The EMA, FDA, and EC met to plan collaboration on pharmaceutical drug development and evaluation.

Checkweighers, metal detectors, x-ray inspectors, leak detectors, headspace analyzers, and optical inspection systems for packaging were demonstrated at INTERPHEX 2015.

FDA released news of the ban of Emcure’s Hinjewadi manufacturing plant after it determined the plant did not meet basic quality standards.

The agency requires early notification of potential drug shortages.

The European Medicines Agency reviews the safety of human papillomavirus vaccines.

The International Conference on Harmonization has moved ICH M7(R1) Addendum to 3 of the ICH process.

Sepha's VisionScan Max can leak-test full production batches of blister packs.

A study now underway on vaccine manufacturing examines the effects of focusing on consistency during manufacturing, instead of post-production testing.

FDA orders unapproved prescription ear drop products with active ingredients removed from the market.

The amendment suggests modifications to the calculation of a drug's average manufacturing price and to the reimbursement rate for infused medications.

Manufacturers and FDA look for innovative strategies to meet accelerated timeframes.

The European Union has a challenging task ahead as it strives to harmonize regulations on advanced therapy medicinal products.

Siegfried Schmitt, principal consultant, PAREXEL, discusses how to handle internal audit reports during inspections.

Heightened regulatory scrutiny of data integrity highlights the need for comprehensive procedural reviews and strategies for managing mission-critical information.
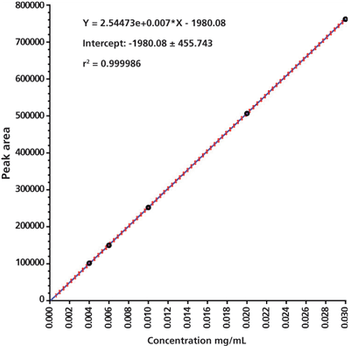
Statistical procedures give statistical answers not analytical judgement.

US Court orders New Jersey drug manufacturer and its president to stop distribution of unapproved and misbranded drugs.

An ISPE guidance document, four years in the making, brings risk-based thinking, statistics, and Lean Six Sigma to cleaning validation.

Richard Johnson, President and CEO of the Parenteral Drug Association (PDA) speaks with Pharmaceutical Technology.

Sue Schniepp, Chair, PDA Regulatory and Quality Advisory Board, and Hal Basemen, Chair, PDA, spoke with Pharmaceutical Technology about resolving drug shortages.

Sue Schniepp, Chair, PDA Regulatory and Quality Advisory Board, and Hal Basemen, Chair, PDA, speak with Pharmaceutical Technology.

The agency gives a limited reprieve to dispensers but requires other trading partners to provide product tracing information.

The US Pharmacopeial Convention hosts compliance seminar at CPhI China 2015.

A recent Ernst & Young survey highlights the challenges facing Indian pharma

Pharmacy associates say more education regarding the transactional requirements of DSCSA is needed.