
The agency has recommended granting marketing authorization for Opdivo.

The agency has recommended granting marketing authorization for Opdivo.

Dicerna Pharmaceuticals announces that FDA granted its primary hyperoxaluria type 1 (PH1) treatment Orphan Drug designation.

The company voluntarily recalls Preservative-Free Bupivacaine HCl Injection, USP due to potential iron oxide particulates.

The agency has released draft guidance on providing regulatory submissions in regards to promotional labeling and advertising materials.

FDA grants Pfizer Breakthrough Therapy designation for its treatment of ROS1-positive non-small cell lung cancer.

Serialization experts will share best practices and practical applications at INTERPHEX 2015.

The European Medicines Agency releases findings from marketing authorization application analysis.

GSK notifies CDC and FDA that it is recalling the remaining doses of its 2014–2015 flu vaccine, Flulaval Quadrivalent Thimerosal-free Pre-Filled Syringes, due to decreased potency.

WHO says that results from clinical trials should be reported within 12 months of completion of the study

FDA extends ANDA rule comment period to June 8, 2015 after requests for more time.

The Ph. Eur. contemplates adding specifications for sub-visible particles in eye drops and eye lotions to its monograph.

At the request of FDA, US Marshals seize unapproved prescription drugs from Florida distributor.

The document serves as guidance for the pharmaceutical industry in data integrity issues and complements existing EU GMP relating to APIs and dosage forms.

This is the first time a single strategy document for both EMA and HMA is presented, reflecting the need for a coordinated approach to address the challenges and opportunities facing the European regulatory system network.

The European Medicines Agency releases guidelines for addressing and reporting risks associated with medication errors.

Agency teams work together to encourage manufacturers to seek approval for previously unapproved drugs.

FDA issues a Warning Letter to Hospira S.p.A. for GMP violations at the company’s Liscate, Italy facility.

Baxter voluntarily recalls select lots of IV solutions due to possible particulate matter.

Industry awaits the final revision of USP General Chapter and the impact it will have on the evaluation of sterile product package integrity.

Quality-by-design tools improve efficiency in scale-up of pharmaceutical processes.

Drug developers understand the importance of early communication with regulators, but is EMA providing enough flexibility and support to companies?
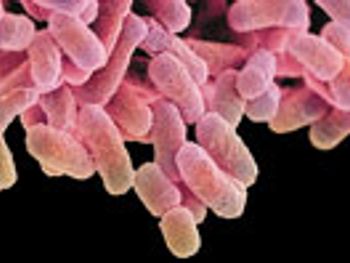
The Human Microbiome Project has increased our understanding of the relationship between humans and microorganisms. The authors offer a new perspective on how this knowledge should be considered in setting standards for pharmaceutical quality control in microbiology.

Process design experimental data and risk assessments are used to predict expected process performance and establish process performance qualification acceptance criteria.

More reliable operations would accelerate product development and prevent drug shortages.

Hope abounds for local drug discovery companies despite challenges at home.