
FDA has approved another indication for Eylea, a treatment for diabetic retinopathy in patients with diabetic macular edema.

FDA has approved another indication for Eylea, a treatment for diabetic retinopathy in patients with diabetic macular edema.

The commission adopts 24 new and 72 revised texts for inclusion in the European Pharmacopoeia.

Emergent BioSolutions announced FDA approval of Anthrasil, an inhalable treatment that targets Anthrax toxins, as well as a contract with BARDA to develop NuThrax, an anthrax vaccine candidate.

The agency provides guidance on determining the need for environmental assessments for gene therapies, vectored vaccines, and viral and microbial products.

Guidance provides labeling standards and an implementation guide for electronic submission of lot distribution reports.

A new machine is designed for inspection of prefilled syringes.

NSF's consensus-based standard incorporates multiple regulatory and industry requirements into a single rigorous standard for the manufacturing and distribution of pharmaceutical excipients.

McNeil-PPC pleads guilty in connection with adulterated infants' and children's over-the-counter liquid medications.

BIO and the Colorado BioScience Association urge Colorado Governor Hickenlooper to sign a bill that will help patients gain access to interchangeable biologic products following FDA approvals.

Astellas Pharma announced that it received FDA approval for its treatment of fatal invasive fungal infections in patients with blood cancers.

In a landmark decision, FDA approved Zarxio, making it the first biosimilar product in the United States.

The Pharma & Biopharma Outsourcing Association announced its support of legislation that would preserve FDA user fees from sequestration.

New rules for tracing drugs through the supply chain and policies for drug compounders change the regulatory landscape.

Scientists and industry experts seek effective preventive therapies to combat global disease.

Proposals to make the decentralized procedure more efficient were discussed at the January 2015 EGA conference.

Siegfried Schmitt, Principal Consultant, PAREXEL International, discusses good engineering practices.

A new technical report guides bio/pharma companies in establishing a risk-based approach for prevention and management of drug shortages.

Assessing risk factors is key to implementing the new ICH Q3D guidelines for elemental impurities.

Cleanability is crucial when choosing components for GMP manufacturing areas.
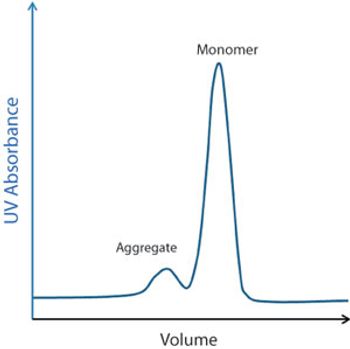
Several chromatographic resins are available for downstream purification.

A General Chapter on mAbs will be published in USP-NF as biologics increase their role in healthcare.

FDA announced that it would postpone a meeting that would be critical for the advancement of Celltrion’s Remicade biosimilar in the US.

FDA approved Actavis’ antibiotic Avycaz designed to combat drug-resistant bacteria.

Johnson & Johnson will pay $2.5 million to a plaintiff who developed gynecomastia after using Risperdal.

The company voluntarily recalls product due to FDA observations of potential sterility problems.