
FDA found violations of cGMP at finished-drug manufacturer in India.

FDA found violations of cGMP at finished-drug manufacturer in India.

Cubist Pharmaceuticals voluntarily recalls certain lots of CUBICIN 500 mg in 10 mL single-use vials because of the presence of particulate matter.

The European system for assessing drugs will be used as a model internationally.

PharmaChk, a portable device being developed to detect substandard medicines, receives $2-million grant.

Biopharma manufacturers must reduce the risk in their complex supply chains

EMA has released the second module of a new guideline on influenza vaccines for a six-month public consultation. The guidance covers the non-clinical and clinical requirements for the development of new influenza vaccines and aims to facilitate the prompt assessment of new vaccines. It follows the publication of a module on the quality requirements.

FDA seeks high quality applications and products to facilitate approvals and reduce safety and supply problems.

The latest revisions to the USP General Chapters <41> Balances and <1251> Weighing on an Analytical Balance aim to ensure weighing accuracy and eliminate unnecessary overtesting by simplifying previous descriptions and reflecting current state-of-the-art weighing practices.

The US federal Drug Quality and Security Act (DQSA) and more specifically the Drug Supply Chain Security Act (DSCSA), referring to Title II of the law, requires phase-in of requirements to prevent counterfeiting. An expert discusses how companies should be preparing to meet the requirements of the DSCSA.

Quality by design, process analytical technology, and high throughput screening have helped drive progress in pharmaceutical development.

The European Union has developed a system for evaluating, approving and monitoring the safety of medicines that has also encouraged innovation.

Quality by Design has initiated a paradigm shift in solid dose pharmaceutical manufacturing.

Global harmonisation would help improve supply-chain security and reduce the flow of falsified and sub-standard medicine into Europe.

The EXCiPACT Certification Scheme ensures patient safety through supplier quality while minimising the audit burden and overall costs for assessing the excipient supply chain.

A breach in the pharmaceutical supply chain is only the tip of the iceberg.

Contract service providers describe how quality by design has influenced a drug sponsor's expectations of suppliers.
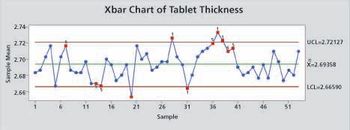
Control charts that are properly constructed and maintained prevent false out-of-control signals and provide a useful method for monitoring a process.

FDA issues a warning letter to Zhejiang Jiuzhou Pharmaceutical Co., Ltd. and its import/export company for multiple CGMP violations and misbranded products.

American Health Packaging announced a voluntary nationwide recall of ibuprofen and oxcarbazepine tablets due to mislabeling on the inner unit dose blister packaging.

Unique Pharmaceuticals has issued a voluntary recall of sterile compounded preparations, but aired concerns about FDA?s recall demand.

Baxter investigates root cause of cellulosic fibers and/or plastics in four lots of IV solutions.

Trifarma cited for significant deviations in data collection and security, and employee training.

Sun Pharma, Forest Pharmaceuticals and West-Ward Pharmaceuticals issue recalls over dissolution issues.

Baxter Healthcare has initiated a nationwide recall of more than 20,000 containers of a pre-mixed beta blocker due to the presence of particulate matter.

The European Medicine Agency details the agency?s recommendations for drug-marketing authorizations for the first half of 2014.