
FDA releases question and answer draft guidance on drug product tracing and licensing requirements.

FDA releases question and answer draft guidance on drug product tracing and licensing requirements.

Impurities, particulates, and a lack of communication are among Hospira's recent violations described in a warning letter from FDA.

Demand for new therapies and vaccines spotlights production challenges.

The recovery of an occasional mold does not merit any particular concern. On the other hand, evidence of mold proliferation indicative of infection of facilities or equipment must be taken seriously and requires the prompt implementation of corrective and preventive actions.

Regulatory agencies in Europe are working to harmonize the marketing approval pathway of generic medicines.
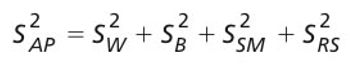
A risk-based guard band surrounds a specification limit and is derived from the uncertainty of the reportable value of the analytical procedure, which includes the uncertainty in the reference standard. The author discusses requirements for generating a reportable value and calculating the associated measurement uncertainty.

Brazil's pharmaceutical industry is optimistic, but is the pharmaceutical market growing steadily or showing signs of instability?

Siegfried Schmitt, Principal Consultant, PAREXEL International, discusses the benefits of automated processes.

Multidirectional collaboration is critical for the new pharma business model; cloud-based information services can offer a communications alternative.

Quality is a matter of culture more than metrics. In this opinion article, the author presents arguments for an FDA Dean's List as a means to nurture a quality culture within the industry.

With a quality-by-design approach, robust processes can help deliver quality product consistently.

USP expresses its support for a consensus-based global approach to the naming of biologics.

FDA report details risk mitigation projects.

Meeting increasing expectations and escalating regulatory requirements to protect patients.

The campaign supports pharmaceutical growth in India and serves to highlight the value proposition that Indian pharmaceuticals present.

GPhA throws its support behind a bill to prohibit companies from using REMS practices to deter competition.

FDA releases guidance on ANDAs and PASs submissions to help applicants avoid deficiencies.

Kurt Lumsden, Director, eCDS Client Services at PAREXEL Informatics, discusses the use of interactive response technologies in investigational product expiry management.

Roche?s RoACTEMRA receives EU approval for use in patients with early rheumatoid arthritis.

PDA surveys are designed to evaluate quality metrics practices at member organizations.

Meggle Group Wasserburg has been granted an EXCiPACT certificate.

Chinese healthcare reforms may be a double-edged sword for foreign companies.
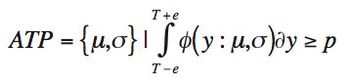
The quality-by-design principles used to control process variability are equally important to measurement systems because process variability includes contributions from measurement system variability. The authors use real-life examples from drug development projects to outline how an understanding of chromatographic measurement system variability might be achieved.

As the pharmacovigilance infrastructure becomes more entrenched in Europe, drug manufacturers are beginning to feel the burden of its high cost.

Industry associations play a strong role in helping the pharmaceutical industry meet challenges.