
USP's revised Chapters 41 and 1251, which became official in December 2013, have new requirements for weighing, including balance calibration and testing.

USP's revised Chapters 41 and 1251, which became official in December 2013, have new requirements for weighing, including balance calibration and testing.

Thirteen companies are accepted for participation in the supply chain program.

Tandem Diabetes Care expands its voluntary recall of select lots of insulin cartridges used with t:slim insulin pump.

NSF-IPEA now can provide excipient auditing and certification.

EMA releases an update on its flu vaccine guidance.

EMA and the European Commission revise Q&A document on implementation of marketing authorization guidelines.

In first official visit to India, FDA commissioner to discuss ongoing collaboration on drug programs.

The Food and Drug Administration plans to issue a number of new guidances in 2014 that will address drug development and manufacturing practices.

FDA urges manufacturers to examine how shape, size, and color may affect patient safety and enhance treatment adherence.

High technology assessments are having an impact on biosimilars development in Europe.

PDA works with FDA to create pharmaceutical quality metrics.

ISPE and PDA take on the challenge of recommending quality metrics.

Siegfried Schmitt, Principal Consultant, PAREXEL International, discusses how human error can be mitigated in pharmaceutical manufacturing.

The biopharmaceutical industry contemplates product innovation within the changing landscape of healthcare.
Non-compliance issues show that users find dealing with computer systems challenging.
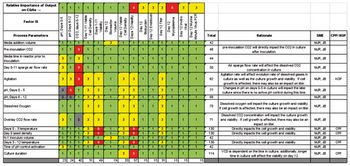
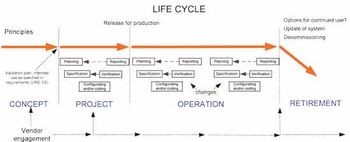
Traditional project decision-making is compared with a QbD approach.

Regis Technologies passes a recent FDA audit with no Form 483 observations.

Some recent high-profile cases of quality issues at Indian manufacturers have given reason to examine manufacturers more closely.

EMA's guidance focuses on the use of pharmacogenomics to improve drug safety monitoring.

NCI launches trial to assess the utility of genetic sequencing to improve patient outcomes.

India has a name when it comes to generic drug development. According to a recent research on patent applications carried out by Withers & Rogers, innovation by Indian pharmaceutical companies has increased over the past few years; however, the quality did not match the standard seen in Europe.

Products enable testing in accordance with methods described in new USP monograph.

FDA adds Ranbaxy's Toansa, India facility to existing consent decree, prohibiting distribution of APIs from that location

Online portal accepts nominations for FDA advisory committee membership.

USP opens expanded Shanghai facility to enhance quality standards for medicines and food ingredients.