
EMA has launched a new version of the EudraGMDP database, which includes the publication of statements of non-compliance with good manufacturing practice.

EMA has launched a new version of the EudraGMDP database, which includes the publication of statements of non-compliance with good manufacturing practice.

Brazil is the first Latin American country to emerge as a global biopharmaceutical collaborator.

Regulators work with manufacturers to improve systems for measuring product performance and manufacturing reliability.

Different approaches for qualification of personnel for visual inspection of residues on equipment surfaces are reviewed.

Kurt Lumden, Director, Client services at PAREXEL's Perceptive Informatics, discusses the management of investigational product temperature excursions.

With SMEs gaining wider recognition as the powerhouse of research and innovation in Europe, regulatory agencies are urging companies to engage with regulators early in the drug-development process.
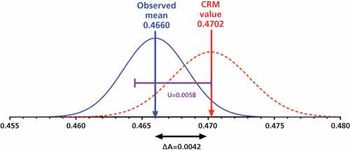
This article takes a statistical look at the calibration requirements for a UV spectrometer.

Abrams Royal Pharmacy is recalling all unexpired lots of sterile products dispensed in the US.

USP is developing and revising distribution standards in response to changes in the global supply chain.

FDA and EMA launch initiative to share bioequivalence inspection information.

FDA has issued a proposed rule to require manufacturers of antibacterial hand soaps and body washes to demonstrate that their products are safe for long-term daily use and more effective than plain soap and water.

Agency issues precautionary recall due to manufacturing fault.

Agency issues precautionary recall due to manufacturing fault.

FDA details efforts to evaluate potential risks from use of nanomaterials in drug products and discuss analytical considerations, impact on stability, safety, and toxicology effects.

OGD is under pressure to improve review operations.

Teva Pharmaceutical Industries releases a financial outlook for 2014 based on two possible scenarios concerning its multiple-sclerosis drug Copaxone (glatiramer acetate).

FDA cites cGMP violations and data-integrity issues and raises concerns over the company's ability to implement a robust and sustainable quality system.

PharmaCheck, a portable tool designed to detect poor-quality medicines, has been selected by Scientific American as one of the magazine?s World Changing Ideas of 2013.

Siegfried Schmitt, principal consultant at PAREXEL, discusses the state of drug manufacturing in India.

The European Union is strengthening its pioneering role in the regulation of biosimilars by further developing the basic rules for determining the levels of compatibility for this group of drugs. There are, however, some key issues that are not easy to resolve, as evident in a recent workshop on biosimilars organized by the European Medicines Agency (EMA).

The rising cost of drug development and the decreasing proportion of drug-naive population in the US and European markets are driving international pharmaceutical companies to consider emerging markets as a location to conduct their clinical trials. Asia stands out among the emerging markets given its double-digit growth rates.

PIC/S reviews deficiencies found during inspections of API manufacturing facilities, harmonizes GMP standards, and provides training for inspectors worldwide.

Brian Galliher of Cook Pharmica discusses visual inspection systems.
The industry lacks an accepted method for establishing a minimum incubation time (MIT) of less than seven days for biological indicators (BIs). The authors propose an MIT method that provides a means for reproducible determination of BI grow-out time.

Industry experts discuss the application, challenges, and benefits of a quality-by-design approach to sterile manufacturing.