
Keith Bader, senior director of technology at Hyde Engineering + Consulting discusses his presentation ?Establishing a design space: cleaning process development and validation,? which will be presented at Interphex 2013

Keith Bader, senior director of technology at Hyde Engineering + Consulting discusses his presentation ?Establishing a design space: cleaning process development and validation,? which will be presented at Interphex 2013

Victor Hernandez of EMD Millipore discusses his upcoming session, "Current continuous process validation program: following FDA current guidelines,? which will be held at Interphex 2013

FDA's Commissioner discusses pharmacy compounding, EMA updates guidance

The 2013 Excipient Information Package (EIP) User Guide is now available for free download from the International Pharmaceutical Excipients Council (IPEC)-Americas.

FDA issues list of 2013 guidance documents.

The Rostov-on-Don Medicines Quality Control Laboratory is the first official medicines control laboratory in Russia to have achieved internationally recognized ISO accreditation.
The authors present topics discussed and conclusions that resulted from the PDA QbD workshop.

Companies risk drowning in alphabet soup if the latest three-letter acronym improvement strategy isn't clearly linked to business strategy.

FDA's requirements for API manufacturers in regards to ICH Q7.

Past IPEC-Americas excipient qualification committee chairs highlight changes to the IPEC guide on certificates of analysis for bulk excipients.

Discussions are underway as the pharmaceutical sector calls for greater consistency in the global monitoring of GMP compliance and quality testing of APIs and finished medicines.

A roundup of regulatory news from across the global pharmaceutical industry.

Industry experts discuss the effect FDA's 2011 process validation guidance has had on industry.

Applying quality-by-design and process analytical technology facilitates process understanding and control of various operations in lyophilization.

QbD paradigm advances process understanding in development and manufacturing.

Vaccine development is benefiting from manufacturing advances and support for global health.

Brazil's major vaccine producer innovates with stem-cell research.

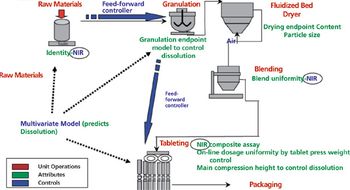
Factors for assessing excipient variability, the associated challenges developers need to address to design and manufacture solid oral drug products, and solutions for such challenges are examined.
The use of various documents and guides by the International Pharmaceutical Excipients Council can facilitate the flow of information among excipient manufacturers, distributors, and users.

An alternative chapter has been added to the European Pharmacopoeia for dosage uniformity.

The European Medicines Agency?s Committee for Orphan Medicinal Products (COMP) is seeking to expand its international cooperation in 2013.

FDA has released a list of more than 50 guidance documents planned for 2013.

The latest news from the pharmaceutical regulatory community.

Siegfried Schmitt, a principal consultant with PAREXEL, discusses how the EU's Falsified Medicines Directive will affect US API production.

Quality assurance of biological products is central to India's good distribution practices guidelines.