
FDA faces budget crunch; Supreme Court hears key cases

FDA faces budget crunch; Supreme Court hears key cases

The author provides a review of FDA's guidance document, Guidance for Industry: Q11 Development and Manufacture of Drug Substances, and its relation to the International Conference on Harmonization's Q11 document and its application to the industry.
A control strategy can maintain a low level of particulates, and thereby a low bioburden, in cleanrooms

FDA will use a new anticounterfeiting tool to detect fake medicines.

FDA issues draft guidance to minimize medication errors.

Third compounding pharmacy recalls products due to FDA inspection.

FDA has released Guidance for Industry: Non-Penicillin Beta-Lactam Drugs: A CGMP Framework for Preventing Cross-Contamination.

FDA inspections of compounding pharmacies manufacturing sterile-drug products lead to voluntary recalls.

An introduction to the upcoming Interphex panel--Lessons Leaned: Successes and Challenges in Implementing Quality by Design.? Moderator: Jennifer Markarian, manufacturing editor, Pharmaceutical Technology. Panelists: John Lepore, senior director of Chemical Process Development and Commercialization for Global Pharmaceutical Commercialization at Merck and Co. Chris Moreton, FinnBrit Consulting Jonathon Thompson, senior manager of Compliance Services Consulting at Invensys Operations Management

FDA's Fiscal Year 2014 budget request includes more than $10 million above the 2012 budget for inspections of products manufactured in China.

FDA inspections of compounding pharmacies manufacturing sterile products reveal non-sterile conditions.

Prefilled-syringe line features automation and novel disinfection techniques.

Company is notified of GMP violations at facility in Catania, Italy.

FDA Releases Guidance on Self-Selections Studies

USP has inaugurated the first satellite site of the USP Spectral Library Global Laboratory Network in China.

Ruling has implications for intellectual property protection for innovator drugs in India.

Latin America's diverse growing market seeks regulatory harmonization.

Industry is moving toward closed-loop control of continuous processing.
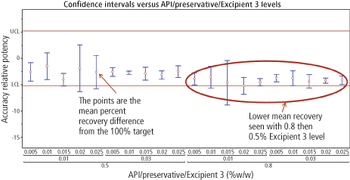
The authors describe a method-validation-by-design (MVbD) approach to validate a method over a range of formulations using both design-of-experiment and quality-by-design principles to define a design space that allows for formulation changes without revalidation.
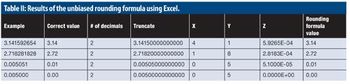
The mysteries of rounding are exposed; strict, unbiased rounding can be applied.

Novartis loses an appeal in seeking patent protection in India for its anticancer drug.

The Thai government is ramping up efforts to promote and develop the biotechnology sector in a bid to enhance its global competitiveness.

Strengthening government control or striving for compliance with international standards?

David Elder, vice-president, technical at PAREXEL, discusses US legislation allows for inspection of generic-drug activities.

Draft guidance combines and supersedes previous guidance documents.