
As a new year begins, what may be on the horizon for 2013 for the biopharmaceutical/pharmaceutical companies and their suppliers? A look ahead into what issues are shaping the industry.
Patricia Van Arnum was executive editor of Pharmaceutical Technology.

As a new year begins, what may be on the horizon for 2013 for the biopharmaceutical/pharmaceutical companies and their suppliers? A look ahead into what issues are shaping the industry.

GlaxoSmithKline partners with the mobile telecommunications company Vodafone to tackle common infectious diseases in Africa.
Process chemists employ a variety of approaches to improve yield, purity, and stereoselectivity.

Additional approvals in December have helped to outpace a recent high set in 2011.

The agency recommends to avoid the use of to dibutyl phthalate and di(2-ethylhexyl) phthalate in CDER-regulated drug and biologic products, including prescription and nonprescription products.

FDA has issued a draft guidance to assist applicants in the submission of summary-level clinical-site data.

MeadWestvaco is recognized at CPhI for its sustainable pharmaceutical packaging.
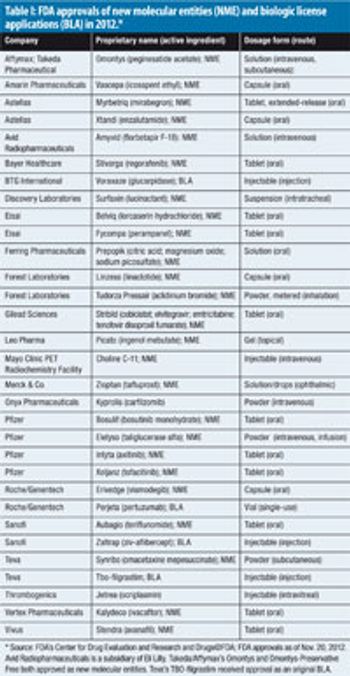
As 2012 comes to a close, Pfizer leads among Big Pharma companies for FDA approvals of new molecular entities and biologics.

As this year comes to a close, a look at the key events shaping the pharmaceutical and biopharmaceutical industry in 2012 and what may be on the horizon for 2013.

Myriad Genetics reports that the US Supreme Court has granted certiorari agreeing to hear the company's gene patentability case.

The company issues voluntary recall of certain lots due to possible contamination with small glass particles.
As 2012 comes to a close, Pfizer leads among Big Pharma companies for FDA approvals of new molecular entities and biologics.

The launch of a global cross-pharmaceutical Investigator Databank follows the formation of TransCelerate BioPharma, another industry collaboration to address challenges in drug development.

Particle-engineering technologies, such as crystal design for crystallization and producting cocrystals, particle-size reduction, and amorphous solid dispersions, help to optimize delivery of a drug.

Fine-chemical manufacturers producers and contract manufacturers announce manufacturing and service expansions at CPhI in Madrid.

Pharmaceutical companies and public health officials gather at the IFPMA Assembly to urge for greater innovation and private and public partnerships to address global health concerns.

Venture-capital funding is up, but initial public offerings still are weak.

Watson Pharmaceuticals has completed its EUR 4.25 billion ($5.4 billion) acquisition of the Actavis Group. The combination creates the world's third largest generic-drug pharmaceutical company, with anticipated pro forma combined 2012 revenues in excess of $8 billion, according to Watson.

The promise of antibody drug conjugates is creating a network of partners among large pharma companies and specialized players.
Pharmaceutical companies and contract service providers adapt strategies and capabilities to reduce costs and accelerate drug-development timelines.

Developing analytical methods and performing related testing is crucial for ensuring the quality of a pharmaceutical product.

Ben Venue Laboratories has resumed production on a limited number of manufacturing lines in the company's Bedford, Ohio, facilities.

The perception of buyers of pharmaceutical ingredients and contract services in how they see market conditions and their own individual business prospects is an important barometer in assessing the market for pharmaceutical outsourcing and ingredients.

The PTE team is currently in Madrid attending numerous meetings and presentations at CPhI and its collocated events. Read the thoughts of our editor on the highlights of the show so far.

As fine-chemical producers, contract manufacturers, and pharmaceutical/biopharmaceutical companies meet this week in Madrid at CPhI Worldwide, what are the key issues on the minds of industry players?

Over the years, CPhI has expanded to cover several areas connected with pharmaceutical manufacturing, but one of the prime focuses of the show is still on ingredients, fine chemicals and intermediates. PharmTech Europe’s exceutive editor, Patricia Van Arnum, tells us more.

Biologic-based drugs are increasingly important in the drug pipelines of pharmaceutical companies.

Merck Serono's Asceneuron is the third company to be spun off from the company's Entrepreneur Partnership Program that was launched to mitigate the impact from recent restructuring at its Geneva site.

A recent review evaluates the nature of global health partnerships and critical factors for their success.

Several Big Pharma companies strengthen their manufacturing presence in Russia.