
A recent book shows the links between synthesis, catalysis, and environmental protection.

A recent book shows the links between synthesis, catalysis, and environmental protection.

Sterile manufacturing may be the next aspect of pharmaceutical manufacturing to consider in the continuous process paradigm.

Equipment qualification is a critical step in ensuring that a product or service is provided accurately and consistently with regards to manufacturing and testing. The time-saving attribute of adding prerequisites to a qualification program, along with the benefit of gaining the ability to track and trend problem areas, make the addition both worthwhile and cost-effective.

The selection of the chiral stationary phase is an important consideration in separating enantiomers when using high-performance liquid chromatography, supercritical fluid chromatography, and simulated moving bed chromatography.

Differential pricing is making more than just an appearance in Asia's pharma market these days.

Brief pharmaceutical news items for September 2008.

This year, the employment survey will acknowledge the industry's best employers.

Identifying the most sustainable packaging for a product is rarely a simple exercise.

FDA is taking several measures to ensure that imported drugs meet manufacturing standards.
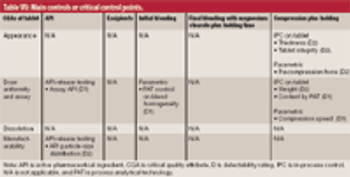
Criticality management combines pharmaceutical product, process, and material knowledge and risk management in one approach, which is reflected in a single document.

With economics and politics in the way, can we defeat the malaria epidemic before it defeats us?
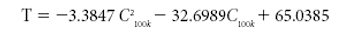
This tutorial paper is meant to aid in dielectric-sensor selection

Fueled by a need to reduce costs and improve efficiencies, continuous processing may be the next paradigm shift in pharmaceutical manufacturing.
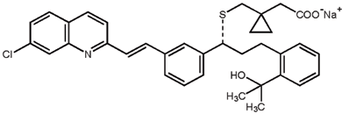
Carbon–hydrogen functionalization, ketone α-alkylation, and biocatalysis are some recent advances in asymmetric synthesis.
Editors' Picks of Pharmaceutical Science & Technology Innovations

Summer deals struck by Novartis and Lilly point to an evolution in contract services.

Operators are hit hard by breakage problems and strike out on FDA inspections.

Are hypersanitation trends a result of scaremongering or a lack of faith in medicine?