
The Sartocon Slice 50 crossflow filter device can be used for membrane screening and small-volume process development.

The Sartocon Slice 50 crossflow filter device can be used for membrane screening and small-volume process development.

The FlexAct BT from Sartorius Stedim Biotech is a point-of-use, leak detection system for single-use bags.

The agency reviews hemophilia A, skin, and diabetes treatments, among others.

The agency completes its risk assessment of the blood cancer treatments.

Patheon launches initial public offering to repay outstanding notes and expenses.

FDA reviewers have found that Sandoz's GP2015, a biosimilar version of Amgen's Enbrel, is highly similar to the original product in terms of purity, safety, and potency.

Cancer cell lines could provide viable pathways to determining how tumors respond to specific anti-cancer drugs.

The agency announced that it has completed the review of the GDUFA backlog one year ahead of schedule.

UPS will expand its clinical trial logistics capabilities to include drug and vaccine development.

The company will collaborate with the Walter Reed Army Institute of Research to co-develop a Zika vaccine candidate.

A Federal Circuit court ruled biosimilar makers must wait 180 days after receiving FDA approval before bringing drugs to market.

The agency says that the routine large-scale compounding of drugs that are exact copies of existing medications undermines the the drug approval process.

The assay will provide information on biosimilar comparability.

Novo Nordisk broke ground on an expanded production plant for insulin in Kalundborg, Denmark.

The draft guidance addresses control of elemental impurities in harmonization with implementation of ICH Q3D guideline.

FDA cited Guangzhou Haishi Biological Technology Co., Ltd. with CGMP violations.

The agency says, for now, it’s business as usual.The European Medicines Agency (EMA) says the future location of the agency will be determined by common agreement between representatives of the Member States, according to a July 6, 2016 statement. Until then, EMA says it will be conducting business as usual, and the outcome of the June 23 referendum will not affect the agency’s operations.

Teligent is expanding its manufacturing and R&D complex in New Jersey.

The acquisition will give Bristol-Myers Squibb full rights to Cormorant’s HuMax-IL8 antibody program.

A study shows high levels of ADAs to infliximab at the beginning of treatment were associated with a poor response later on.

International-domestic pharma partnerships will drive next wave of growth in China, according to new CPhI report on China.
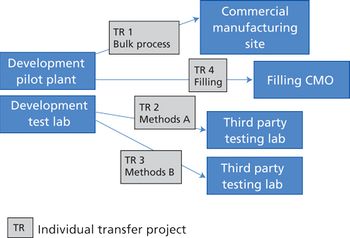
Integrating quality and compliance with technology transfer and careful project management are key in starting up a facility and launching a biologic drug.
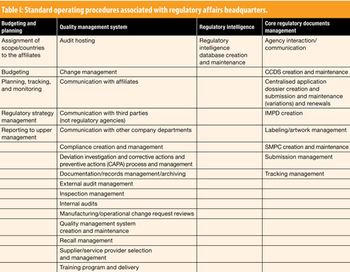
Siegfried Schmitt, Principal Consultant, PAREXEL International, discusses standard operating procedures for the regulatory affairs department.

CPhI Pharma Awards seek nominations for excellence in development and manufacturing.

The company expanded its services to include oligonucleotide API development and manufacturing and received approval for its Caponago manufacturing facility.

Evonik will acquire Transferra for an undisclosed amount, and the transaction is scheduled to close at the end of July 2016

A study by MilliporeSigma and the Economist Intelligence Unit reviews growth drivers and approaches to mitigate risk.

The drug received breakthrough therapy and orphan drug designation as a monotherapy for the treatment of chronic graft-versus-host-disease.

Califf appointed Richard Pazdur as acting director of the Oncology Center of Excellence under the Cancer Moonshot Initiative.