
The company will install new vial-filling and lyophilization capacity, including an isolator-based filling machine. The new filling equipment will result in a five-fold increase in the company's current filling capacity.

The company will install new vial-filling and lyophilization capacity, including an isolator-based filling machine. The new filling equipment will result in a five-fold increase in the company's current filling capacity.
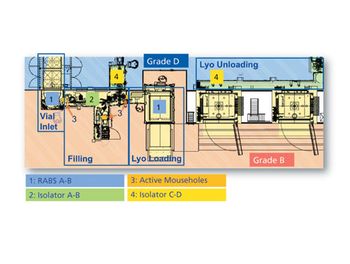
The design of Baxter BioPharma Solutions’ aseptic filling lines provides a case study in customizing containment systems for multi-product lines

The collaboration will focus on methods to eradicate cancer stem cells

The acquisition will add to the company's cell therapy base and potential impact in treating rare diseases.

An MIT-developed system uses microbes for manufacturing small amounts of vaccines and other therapies.

The new materials handling laboratory will be used for storage, dispensing, inventory management, and distribution of non-GMP materials.

Congressional partisanship creates noise, but no funding for Zika virus research.

Galvani Bioelectronics will be based in Stevenage, UK, and headed by Kris Famm, GSK's vice president of bioelectronics R&D

Amgen discussed the drug-delivery approaches to two of its biologics in a recent second-quarter earnings call.

NIST’s monoclonal antibody reference material can be used as a standard for biopharmaceutical analytical quality control.

The investment will be spread across three sites: Barnard Castle in County Durham; Montrose, Scotland; and Ware, Hertfordshire.

The move follows a warning from FDA wherein FDA cited ALK-Abello with numerous manufacturing violations.

FDA and industry seek speedy Congressional approval of new user fee plan.

The Phase I trial will test Bavarian Nordic’s vaccine, which is manufactured using a vaccine vector based on smallpox.The recent resurgence of yellow fever incidences over the past six months has prompted health officials to ramp up the fight against the virus. Like the Zika virus, yellow fever is transmitted primarily through the bite of infected female Aedes aegypti mosquitoes.

On July 27, 2016, John C. Lechleiter, PhD, chairman, president, and CEO of Eli Lilly, announced his retirement from the company. This decision is effective December 31, 2016, however, Lechleiter will remain on the company’s board of directors until May 31, 2017 as a non-executive chairman. Lechleiter joined the company in 1979 as a senior organic chemist in process R&D. He has served as CEO of Eli Lilly since April 2008.

The affinity purification company will set up shop at the former Merck & Co./GlycoFi facility in the Dartmouth Regional Technology Center in New Hampshire.

The project involves installation of a small-scale pressured agitated nutsche filter dryer in glass, integrated in a high-containment isolator to achieve an occupational exposure limit of less than 1 microgram per meter cube, 8-hour time weighted average.

A new era has begun to address the deadly innovation gap in tackling the antimicrobial resistance crisis.

CDMO Saneca Pharma has sold registration dossiers for more than 20 pharmaceutical products to Xantis Pharma and will hold an exclusive five-year contract manufacturing agreement for the products with Xantis Pharma.

The therapeutic candidate AZD8601is an investigational mRNA-based therapy that will be tested for its ability to regulate the protein that influences vascular tissue growth.

The mAb, in combination with lenalidomide and dexamethasone, or bortezomib and dexamethasone, was granted a Breakthrough Therapy Designation from FDA for the treatment of multiple myeloma.

On July 25, 2016 Kite Pharma entered into an agreement with the University of California, Los Angeles, (UCLA) to advance development of off-the-shelf allogenic T-cell therapies from renewable pluripotent stem cells. The company entered into an exclusive license agreement with UCLA for an artificial thymic organoid (ATO) cell culture system. The ATO replicates the human thymic environment to support efficient ex vivo differentiation of T-cells from primary and reprogrammed pluripotent stem cells.

The companies filed a BLA for romosozumab, an investigational monoclonal antibody for the treatment of osteoporosis.

The vaccine candidate has also won a Priority Medicines (PRIME) status from EMA.

The companies will test the efficacy of Rova-T in combination with Opdivo, and Opdivo + Yervoy as a treatment for extensive-stage small cell lung cancer.

The agency will review Mylan and Biocon’s biosimilar to pegfilgrastim.

The agency recommends suspending drugs developed with bioequivalence studies performed at Semler Research Centre Private Ltd, Bangalore, India.

The agency has recommended granting marketing authorization in the EU for Truvada.

The company will expand its service offerings to include oligonucleotide API development and manufacture.

The Nexera-i MT from Shimadzu incorporates two flow lines, which allow for both UHPLC and HPLC analysis.