
FDA approved Probuphine, the first buprenorphine implant approved in the United States for the treatment of opioid dependence.

FDA approved Probuphine, the first buprenorphine implant approved in the United States for the treatment of opioid dependence.

The company received a warning letter at its Latina facility in Sermoneta, Italy following a May 2015 inspection.

The agency publishes draft guidance on assessing the adhesion of transdermal delivery systems and topical patches.

Lagarde will be based out of the company’s Boston office and will lead Patheon’s global corporate and cross-enterprise operations.

PharmTech sat down with an intellectual property lawyer to examine how companies are protected when they engage in activities where sharing of trade secrets must occur.

Samsung Bioepis and partner Biogen announced on May 30 that the European Commission approved Flixabi for the treatment of six inflammatory conditions.

Xellia, a generic anti-infective drug manufacturing company, is constructing a laboratory services building at its manufacturing site in Budapest, Hungary.

The United States Geological Survey study detected one or more pharmaceuticals in 59 streams throughout the Southeast United States.

The AAPS Foundation presented five graduate students each with a $10,000 fellowship for their research in the pharmaceutical sciences.

The agency cited Tai Heng Industry with CGMP deviations, including failure to ensure data integrity.

Sandoz is seeking approval for the same indications as Roche’s reference product MabThera.

The new 500-meter-square warehouse will hold incoming materials, including already micronized highly potent substances and cytotoxic products.

Cell and Gene Therapy Catapult and the Australian CRC for Cell Therapy Manufacturing to will collaborate on a project to test technology for T-cell stimulation and expansion.

The company will offer a standard serialization solution across 14 locations in Europe and more than 70 product lines.

In the largest-ever report of its kind, drugs entering clinical development in Phase I were found to have only a 1 in 10 chance of FDA approval.

Colorcon Acquired BASF’s Kollicoat IR Coating System product line, which includes currently customer business, and inventory.

FDA accepted for review Samsung Bioepis’ BLA for SB2, a biosimilar to Remicade (infliximab).

FDA cited BBT Biotech GMBH for failing to comply with current good manufacturing practice in its German API manufacturing facility.

The companies have launched VEGF 165, their first joint product for cell culture applications.

W.R. Grace & Co. will sell its chromatography product lines, which includes chromatography instruments, columns, and other related products.

Sanofi announced that it is making several changes to its executive committee, effective June 1, 2016.

The draft guidance helps companies design new treatments of chronic obstructive pulmonary disease.

A new study in Nature Communications explores how to remove the bulk of the soaps that are added to injectables to make hydrophobic drugs more soluble.

Genentech received accelerated approval for its immunotherapy atezolizumab for the treatment of bladder cancer.

Celgene will pay Agios $200 million to lead exploratory, research, drug discovery, and early development work for metabolic immuno-oncology.
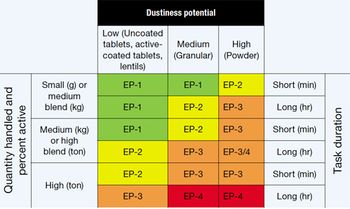
Safe handling of HPAPIs requires determining exposure potential and selecting appropriate containment strategies.

The company’s method reduces the time required to crystallize antibodies from weeks to one day.

The company recalls products due to sterility concerns.

The NIH and partners will launch a large-scale clinical trial in South Africa to evaluate the effectiveness of an HIV vaccine regimen

The agency detailed its 2015 achievements in an annual report.